Summary
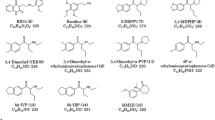
In this paper the isolation and identification of 12 compounds as impurities in illicit 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxymethamphetamine (MDMA) is reported. Isolation of these substances is performed by preparative TLC, while identification is performed by using mass spectrometry and1H-NMR spectroscopy. A simple and rapid method for detection of these impurities in seized MDA and MDMA samples is described. The identification of the impurities can provide numerous points on which to base comparative analysis of different exhibits.
Zusammenfassung
Es wird die Isolierung und Identifizierung von 12 Synthesebeiprodukten in illegalem 3,4-Methylendioxyamphetamin (MDA) und 3,4-Methylendioxymethamphetamin (MDMA) beschrieben. Die Isolierung dieser Substanzen wird durch präparative Dünnschichtchromatographie erreicht, während die Identifizierung durch GC/MS und1H-NMR Spektroskopie erfolgt. Anschließend wird eine einfache und schnelle Methode, diese Substanzen in beschlagnahmten MDA- bzw. MDMA-Proben nachzuweisen, vorgestellt. Der Nachweis dieser Substanzen kann eine wichtige Hilfe dabei sein, eine vergleichende Analytik verschiedener Proben vorzunehmen.
Similar content being viewed by others
References
Mannich C, Jacobsohn W (1910) Über Oxyphenylalkylamine und Dioxyphenylalkylamine. Ber Dtsch Chem Ges 43:189
Anon. German Patent 274, 350, (1914), issued to, E. Merck in Darmstadt, May 16, 1914
Crossley FL, Moore ML (1944) Studies on the Leuckart Reaction. J Org Chem 9:529–536
Ingersoll AW, Brown JH, Kim CK, Beauchamp WD, Jennings R (1936) Extension of the Leuckart Syntheses of amines. J Am Chem Soc 58:1808–11
Novelli A (1939) Symphathomimetics. Preparation of nitrogen substituted β-phenylisopropylamines. J Am Chem Soc 61:520
Fujisawa T, Okada M, Deguchi Y (1956) 1-(β-diethylamino-ethoxyphenyl)-3-methyl-3,4-dihydro-6,7-methylenedioxyisoquinoline Japanese Patent 8573; abstracted: (1958) Chem Abstr 52:11965b
Frank RS (1983) The clandestine drug laboratory situation in the United States. J Forensic Sci 28:18–31
v.d. Ark AM, Verweij AMA, Sinnema A (1978) Weakly basic impurities in illicit amphetamine. J Forensic Sci 23:693–700
Lukaszewski T (1978) Spectroscopic and chromatographic identification of precursors, intermediates and impurities of 3,4-methylenedioxyamphetamine syntheses. J Assoc Off Anal Chem 61:951–967
Alexander ER, Misegades AL (1948) A low pressure reductive alkylation method for the conversion of ketones to primary amines. J Am Chem Soc 70:1315–1316
Haskelberg L (1948) Aminative reduction of ketones. J Am Chem Soc 70:2811–2812
Braun U, Shulgin AT, Braun G (1980) Centrally active N-substituted analogs of 3,4-methylenedioxyamphetamine. J Pharm Sci 69:192–195
Magidson OY, Garkusha GA (1941) Synthesis of β-phenylisopropylamines. J Gen Chem (U.S.S.R) 11:339–343; abstracted: (1941) Chem Abstr 35:5868
Aonuma S, Ilama T, Kametani T (1955) The effect of benzylamine derivatives on blood vessels and heart of frog. Jpn J Pharm Chem 27:607–611 abstracted: (1956) Chem Abstr 50: 13292 g
Herbst RM, Manske RH (1936) Methylbenzylketon (Phenylacetone). Org Syn Coll 2:389–391
Bourn AJR, Gillies DG, Randall EW (1964) Cis-trans isomerism in formanilide. Tetrahedron 20:1811–1818
La Planche LA, Rogers MT (1963) Cis and trans configurations of the peptide bond in N-monosubstituted amides by nuclear magnetic resonance. J Am Chem Soc 86:337–341
Verweij AMA (1990) Clandestine manufacture of MDMA by low pressure reductive amination. A mass spectrometric study of some reaction mixtures. Forensic Sci Int 45:91–96
Verweij AMA (1991) Verunreinigungen in illegalem Amphetamin. Arch Kriminol 188:155–158
Eight Peak Index of Mass Spectra (1983) The Mass Spectrometry Data Centre, the Royal Society of Chemistry: Nottingham
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bohn, M., Bohn, G. & Blaschke, G. Synthesis markers in illegally manufactured 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine. Int J Leg Med 106, 19–23 (1993). https://doi.org/10.1007/BF01225019
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01225019