Abstract
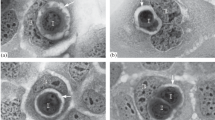
The induction of cell death along with cell-cycle arrest is one of the foremost mechanisms regulating cell growth. In the human breast carcinoma cell line MCF-7 we investigated two chemotherapeutic agents, the antiestrogen tamoxifen and the DNA-damaging drug cisplatin, for the relative contribution of these mechanisms to growth inhibition in culture. Growth kinetics and flow cytometry confirmed that tamoxifen at 1 μM acts mainly by arresting cells in the G0/G1 phase of the cell cycle. Compared to untreated controls, only a few more cells were detached from the monolayer and dead after a 5-day incubation. On the other hand, cisplatin at 1 μM did not induce the well-defined G2/M-arrest reported for other cell types, but resulted in a marked increase in the rate of cell death. A morphological feature observed, especially with cisplatin-treated MCF-7 cells, was the formation of numerous micronuclei (in up to 30% of the cells) and an increase in the number of binucleate cells (up to 20%). In both tamoxifen- and cisplatin-treated cultures, cell death appeared to occur by apoptosis, as indicated morphologically by cellular and nuclear shrinkage accompanied by DNA-condensation and ultimately the formation of DNA containing apoptotic bodies. However, no internucleosomal DNA degradation or endogenous endonuclease activity could be detected in the cells of the monolayer or in the mainly dead and detached cells of the culture supernatant. DNA fragmentation was only observed when isolated MCF-7 nuclei were incubated with exogenous endonucleases. However, as determined by reverse transcriptase/polymerase chain reaction amplification, MCF-7 cells do express the mRNA for DNase I, an endonuclease known to be involved in apoptosis. Thus, apoptosis is part of the growth-inhibitory process and occurs without apparent internucleosomal DNA fragmentation in MCF-7 cell cultures.
Similar content being viewed by others
Abbreviations
- FCS :
-
fetal calf serum
- PBS :
-
phosphate-buffered saline
References
Almendral JM, Sommer D, MacDonald-Bravo H, Burckhardt J, Perera J, Bravo R (1988) Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol 8:2140–2148
Bardon S, Vignon F, Montcourrier P, Rochefort H (1987) Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res 47:1441–1448
Barry MA, Eastman A (1993) Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys 300:440–450
Butler WB (1984) Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal Biochem 141:70–73
Eastman A (1990) Activation of programmed cell death by anticancer agents: cisplatin as model system. Cancer Cells 2:275–280
Evans DL, Dive C (1993) Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res 53:2133–2139
Gaido ML, Cidlowski JA (1991) Identification, purification, and characterization of a calcium-dependent endonuclease (NUC 18) from apoptotic rat thymocytes. J Biol Chem 266:18580–18585
Geier A, Weiss C, Beery R, Haimsohn M, Hemi R, Malik Z, Karasik A (1995) Multiple pathways are involved in protection of MCF-7 cells against death due to protein synthesis inhibition. J Cell Physiol 163:570–576
Hickman JA (1992) Apoptosis induced by anticancer drugs. Metastasis Rev 11:121–139
Kelland LR (1994) The molecular basis of cisplatin sensitivity/resistance. Eur J Cancer 30A:725–727
Köpf-Maier P, Wagner W, Liss E (1983) Induction of cell arrest at G1/S and in G2 after treatment of Ehrlich ascites tumor cells with metallocene dichlorides and cis-platinum in vitro. J Cancer Res Clin Oncol 106:44–52
Kyprianou N, English HF, Davidson NE, Isaacs JT (1991) Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res 51:162–166
Majone F, Tonetto S, Soligo C, Panozzo M (1992) Identification of kinetochores and DNA synthesis in micronuclei induced by mitomycin C and colchicine in Chinese hamster ovary cells. Teratogenesis Carcinog Mutagen 12:155–166
Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M (1993) Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 12:3679–3684
Osborne CK, Boldt DH, Clark GM, Trent JM (1983) Effects of tamoxifen on human breast cancer cell cycle kinetics: accumulation of cells in early G1 phase. Cancer Res 43:3583–3585
Otto AM, Faderl M, Schönenberger H (1991) Dissociation of estrogenic and cytotoxic properties of an estrogen receptor binding platin complex in human breast cancer cell lines. Cancer Res 51:3217–3223
Pacchierotti F, Bassani B, Leopardi P, Zijno A (1991) Origin of aneuploidy in relation to disturbances of cell-cycle progression. II. Cytogenetic analysis of various parameters in mouse bone marrow cells after colchicine or hydroquinone treatment. Mutagenesis 6:307–311
Paddenberg R, Wulf S, Weber A, Heimann P, Beck LA, Mannherz HG (1996) Internucelosomal DNA fragmentation in cultured cells under conditions reported to induce apoptosis may be caused by mycoplasmal endonucleases. Eur J Cell Biol 71:105–119
Peitsch MC, Polzar B, Stephan H, Crompton T, MacDonald HR, Mannherz HG, Tschopp J (1993) Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J 12:371–377
Peitsch MC, Mannherz HG, Tschopp J (1994) The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol 4:37–41
Peyrot V, Briand C, Momburg R, Sari JC (1986) In vitro mechanism study of microtubule assembly inhibition bycis-dichlorodiammine-platinum(II). Biochem Pharmacol 35:371–375
Polzar B, Zanotti S, Stephan H, Rauch F, Peitsch MC, Irmler M, Tschopp J, Mannherz HG (1994) Distribution of deoxyribonuclease I in rat tissues and its correlation to cellular turnover and apoptosis (programmed cell death). Eur J Cell Biol 64:200–210
Ren L, Zhang H, Yang J, Zhang Z (1993) A sequential study on the use of the cytokinesis-block micronucleus method in mouse splenocytes. Mutat Res 301:223–227
Roberts JJ, Fraval HNA (1978) The interaction of antitumor platinum compounds with cellular DNA in cultured cells and animal tissues: relationship to DNA cellular repair processes. Biochimie 60:869–877
Rodilla V, Pellicer JA, Pertusa J, Mothersill C (1990) Induction of micronucleated and binucleate cells in Chinese hamster ovary (CHO) cells bycis-diamminedichloroplatinum (II): a morphological and morphometric study. Mutat Res 241:115–124
Rodilla V, Pellicer JA, Serrano A, Pertusa J (1993) Possible relationship between micronucleated and binucleated cells induced by cisplatin in cultured CHO cells. Mutat Res 291:35–41
Rosenberg B (1973) Platinum coordination complexes in cancer therapy. Naturwissenschaften 60:399–406
Segal-Bendirdjian E, Jacquemin-Sablon A (1995) Cisplatin resistance in a murine leukemia cell line is associated with a defective apoptotic process. Exp Cell Res 218:201–212
Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL (1990) Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci USA 87:9188–9192
Sledge GWJ (1992) Cisplatin and platinum analogues in breast cancer. Semin Oncol 19:78–82
Sorenson CM, Eastman A (1988a) Influence ofcis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excicion repair proficient and deficient Chinese hamster ovary cells. Cancer Res 48:6703–6707
Sorenson CM, Eastman A (1988b) Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand break. Cancer Res 48:4484–4488
Sorenson CM, Barry MA, Eastman A (1990) Analysis of events associated with cell cycle arrest at G2 and cell death induced by cisplatin. J Natl Cancer Inst 82:749–755
Stopper H, Eckert I, Schiffmann D, Spencer DL, Caspary WJ (1994) Is micronucleus induction by aneugens an early event leading to mutagenesis? Mutagenesis 9:411–416
Styles JA, Davies A, Lim CK, Matteis F de, White INH, Yuan Z-X, Smith LI (1994) Genetoxicity of tamoxifen-tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis 15:5–9
Sutherland RL, Hall RE, Taylor IW (1983) Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res 43:3998–4006
Sutherland RL, Green MD, Hall RE, Reddel RR, Taylor IW (1984) Tamoxifen induces accumulation of MCF-7 human mammary carcinoma cells in the G0/G1 phase of the cell cycle. Eur J Cancer Clin Oncol 19:615–621
Taylor CM, Blanchard B, Zava DT (1984) Estrogen receptor-mediated and cytotoxic effects of the antiestrogens tamoxifen and 4-hydroxytamoxifen. Cancer Res 44:1409–1414
Taylor IW, Hodson PJ, Green MD, Sutherland RL (1983) Effects of tamoxifen on cell cycle progression of synchronous MCF-7 human mammary carcinoma cells. Cancer Res 43:4007–4010
Wärri AM, Huovinen RL, Martikainen PM, Härkönen PL (1993) Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. J Natl Cancer Inst 85:1412–1418
Wright M, Lacorre-Arescaldino I, Macquet JP, Daffe M (1984) Induction of polyploid nuclei in the plasmodium ofPhysarum polycephalum by platinum antitumor compounds. Cancer Res 44:777–783
Wyllie AH, Kerr FR, Currie AR (1980) Cell death: the significance of apoptosis. Int Rev Cytol 68:251–305
Wyllie AH, Morris RG, Smith AL, Dunlop D (1984) Chromatin cleavage in apoptosis: Association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol 142:67–77
Yasuda T, Mizuta K, Ikehara Y, Kishi K (1989) Genetic analysis of human deoxyribonuclease I by immunoblotting and the zymogram method following isoelectric focussing. Anal Biochem 183:84–88
Zakeri Z, Bursch W, Tenniswood M, Lockshin R (1995) Cell death: programmed, apoptosis, necrosis, or other? Cell Death Differ 2:87–96
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Otto, A.M., Paddenberg, R., Schubert, S. et al. Cell-cycle arrest, micronucleus formation, and cell death in growth inhibition of MCF-7 breast cancer cells by tamoxifen and cisplatin. J Cancer Res Clin Oncol 122, 603–612 (1996). https://doi.org/10.1007/BF01221192
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01221192