Summary
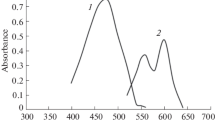
ECRC forms violet coloured chelates with aluminium(III) and gallium(III) with λmax 540 nm and 550 nm respectively at pH 4.5 and 3.5. The metal ligand ratio in both the cases has been found to be 1∶1. The chelates of aluminium and gallium are stable between pH 3.5 to 5.5 and pH 2.7 to 6.0 respectively. The apparent stability constants are 104.9 (pH 4.5) and 104.8 (pH 3.8) respectively for the aluminium and gallium chelates at 25°. The range of concentration for adherence toBeer's law, the effective range of photometric determination and the molar absorptivities are 0.27 to 0.97 p. p. m.; 0.18 to 0.97 p. p. m. and 3.20 · 104 for aluminium, and 0.093 to 2.52 p. p. m.; 0.46 to 2.52 p. p. m. and 2.20 ·104 for gallium respectively.
Zusammenfassung
Eryochromcyanin RC bildet mit Al(III) und Ga(III) violett gefärbte Chelate, die bei pH 4,5 bzw. 3,5 Absorptionsmaxima bei 540 bzw. 550 run haben. In beiden Fällen ist das Verhältnis Metall: Ligand=1∶1. Die Chelate sind zwischen pH 3,5 und 5,5 bzw. zwischen pH 2,7 und 6,0 stabil. Die entsprechenden Konstanten lauten 104,9 (pH 4,5) bzw. 104,8 (pH 3,8) bei 25°. Bei Al liegt der Gültigkeitsbereich für das Beersche Gesetz zwischen 0,27 und 0,97 ppm; für die photometrische Bestimmung zwischen 0,18 und 0,97 ppm. Die molare Extinktion beträgt 3,20 · 104. Die entsprechenden Werte für Gallium lauten: 0,093 bis 2,52 ppm; 0,46 bis 2,52 ppm und 2,20 · 104.
Similar content being viewed by others
References
A. K. Babko andP. P. Kish, Zhur. Analit. Khim.17, 693 (1962).
S. C. Shrivastawa andA. K. Dey, Doctoral thesis ofS. C. Shrivastawa, Allahabad 1966.
K. N. Munshi andA. K. Dey, D. Sc. thesis ofK. N. Munshi, Allahabad 1966.
T. Millner, Z. analyt. Chem.113, 83 (1938).
P. U. Sakellaridis andB. S. Roufogahis, Chim. Chronika (Athens, Greece)27, 123 (1962).
P. Job, C. r. acad. sci., Paris180, 928 (1925); Ann. chim. (10),9, 113 (1928).
A. E. Harvey andD. L. Manning, J. Amer. Chem. Soc.72, 4488 (1950).
S. K. Banerji andA. K. Dey, Proc. Symp. Chem. Coord. Compounds, Agra 1959,2, 198 (1960).
A. K. Mukherji andA. K. Dey, Analyt. Chim. Acta18, 324 (1958).
J. H. Toe andA. L. Jones, Ind. Eng. Chem., Analyt. Ed.16, 111 (1944).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garg, V.C., Shrivastawa, S.C. & Dey, A.K. Determination of aluminium and gallium: Chelate formation of trivalent aluminium and gallium with eriochrome cyanine RC. Mikrochim Acta 57, 668–672 (1969). https://doi.org/10.1007/BF01216473
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01216473