Abstract
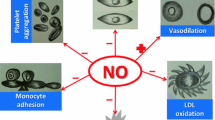
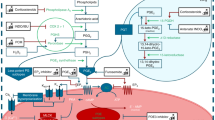
The role of eicosanoids, arachidonic acid (AA) metabolites, in blood pressure regulation under physiological and pathological conditions during the perinatal period is still under investigation. This review focuses on the synthesis and catabolism of some vasoactive AA metabolites by fetal, neonatal and placental cells, and on the vascular responses of the fetus and neonate to prostanoids and to the inhibitors of their synthesis. Vasodilator prostaglandins, PGE2 and prostacyclin (PGI2), increase steadily during pregnancy, while thromboxane A2 (TXA2), a potent vasoconstrictor, remains low during pregnancy, increasing only shortly before delivery. TXA2 participates in the closure of umbilical vessels and ductus arteriosus. In pregnancy-induced hypertension, increase in the synthesis of TXA2 occurs early during pregnancy. Decrease in the catabolism of AA precedes the onset of hypertension in the developing spontaneously hypertensive rat. In newborn piglets, platelet-activating factor, vasoconstrictor prostaglandins and leukotriene D4 have a marked constrictor effect on the pulmonary circulation and induce pulmonary hypertension, without affecting the systemic blood pressure. Although the role of AA metabolites in the regulation of haemodynamics during the perinatal period is not fully understood, it is apparent that several eicosanoids modulate the action of hormones and vasoactive agents.
Similar content being viewed by others
References
Reyes JL, Meléndes E, Escalante BA, Namorado MC (1989) Effect of synthesis inhibitors of thromboxane A2 and prostaglandin E2 on the regulation of sodium and water. J Pharmacol Exp Ther 251: 694–699
Rosenkrans CF Jr, Paria BC, Davis DL, Milliken G (1992) Synthesis of prostaglandins by pig blastocysts cultured in medium containing estradiol or catechol estrogen. Prostaglandins 43: 309–319
Novy MJ, Cook MJ, Manaugh L (1974) Indomethacin block of normal onset of parturition in primates. Am J Obstet Gynecol 118: 412–416
Karim SMM (1978) On the use of blockers of prostaglandin synthesis in the control of labor. Adv Prostaglandin Thromboxane Res 4: 301–306
Coceani F, Olley PM, Bodach E (1976) Prostaglandins: a possible regulator of muscle tone in the ductus arteriosus. Adv Prostaglandin Thromboxane Res 1: 417–424
Sharpe GL, Larsson KS, Thalme B (1974) Studies of closure of the ductus arteriosus. XII. In utero effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins 9: 585–596
Sharpe GL, Thalme B, Larsson KS (1975) Studies on closure of the ductus arteriosus. XI. Ductal closure in utero by a prostaglandin synthetase inhibitor. Prostaglandins 10: 363–368
Arcilla RA, Thilenius OG, Ranniger K (1969) Congestive heart failure from suspected ductal closure in utero. J Pediatr 75: 74–78
Wiqvist N, Lundström V, Green K (1975) Premature labor and indomethacin. Prostaglandins 10: 515–526
Pace-Asciak CR (1976) Decreased renal prostaglandin catabolism precedes onset of hypertension in the developing spontaneously hypertensive rat. Nature 263: 510–511
Chemtob S, Beharry K, Rex J, Varma DR, Aranda JV (1990) Changes in cerebrovascular prostaglandins and thromboxane as a function of systemic blood pressure. Cerebral flow autoregulation of the newborn. Circ Res 67: 674–682
Van Geet KC, Eggermont E, Vermylen J (1992) Urinary TXB2 metabolites as indicator of in vivo platelet activation in the neonate (abstract). Proceedings of the 8th International Conference on Prostaglandins and Related Compounds, Montreal, Canada, 26–31 July, Montreal, Can., p. 29
Seyberth HW, Rascher W, Schweer H, Kuhl PG, Mehls O, Scharer K (1985) Congenital hypokalemia with hypercalciuria in preterm infants: a hyperprostaglandinuric tubular syndrome different from Bartter syndrome. J Pediatr 107: 694–701
Seyberth HW, Königer SJ, Rascher W, Kuhl PG, Schweer H (1987) Role of prostaglandins in hyperprostaglandin E syndrome and in selected renal tubular disorders. Pediatr Nephrol 1: 491–497
Seyberth HW (1989) Prostaglandin metabolism in congenital tubular disorders. In: Schärer K. (ed.) Growth and Endocrine changes in children and adolescents with chronic renal failure. Pediatr Adolesc Endocrinol, Basel, Karger, 20: 147–150
Altken MA, Rice G, Brennecke S (1992) Relative abundance of human placental phospholipase A2 messenger RNA in late pregnancy. Prostaglandins 43: 361–370
Izhar M, Pasmanik M, Marcus S, Shemesh M (1992) Dexamethasone inhibition of cyclooxygenase expression in bovine term placenta. Prostaglandins 43: 239–254
López-Bernal A, Hansell DJ, Khong TY, Keeling JW, Turnbull AC (1990) Placental leukotriene B4 release in early pregnancy and in term and preterm labour. Early Hum Dev 23: 93–99
Bennet PR, Elder MG (1992) The mechanisms of preterm labour, the interaction between amnion cells and leukocytes in the metabolism of arachidonic acid. Prostaglandins 43: 87–98
Terragno NA, Terragno DA, McGiff JC (1976) The role of prostaglandins in the control of uterine blood flow. In: Lindheimer MD, Katz AI, Zuspan FP (eds) Hypertension in pregnancy. Wiley, New York, pp 391–398
Mitchell MD, Romero RJ, Lepera R, Rittenhouse L, Edwin SS (1990) Actions of endothelin-1 on prostaglandin production by gestational tissues. Prostaglandins 40: 627–635
Mitchell MD (1991) Endothelins in perinatal biology. Semin Perinatol 15: 79–85
Krzeski R, Long W, Katayama, Henry W (1992) Hemodynamic effects of endothelin-1 in the newborn piglet. Influence on pulmonary and systemic vascular resistance. J Cardiovasc Pharmacol 17 [Suppl 7]: S322-S325
Wang Y, Coceani F (1992) Prostaglandin and nitric oxide: evidence for a role in the dilatation of the pulmonary circulation at birth (abstract). Proceedings of the 8th International Conference on Prostaglandin and Related Compounds, Montreal, Canada, 26–31 July, Montreal, Can., p. 29
Reyes JL, Nava E, Namorado MC (1992) Receptor-mediated effect of a synthetic thromboxane-analogue on cytosolic calcium in isolated proximal tubules. Prostaglandins 44: 145–154
Sung RYT, Yin JA, Loong EPL, Fok TF, Lau J (1990) Topical prostaglandin E2 gel for cervical ripening and closure of the ductus arteriosus in the newborn. Arch Dis Child 65: 703–704
Rankin JHG, Phernetton TM (1976) Circulatory responses of the near-term sheep fetus to prostaglandin E2. Am J Physiol 231: 760–765
Novy MJ, Piasecki G, Jackson BT (1974) Effect of prostaglandins E2 and F2alpha on umbilical blood flow and fetal hemodynamics. Prostaglandins 5: 543–555
Weiner CP, Robillard JE (1989) Effect of acute intravascular volume expansion on human fetal prostaglandin concentrations. Am J Obstet Gynecol 161: 1494–1497
Leuschen MP, Ehrenfried JA, Willet LD, Schroder KA, Bussey ME, Bolam DL, Goodrich PD, Nelson RM (1991) Prostaglandin F1α levels during and after neonatal extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 101: 148–152
Dunn M (1987) The role of arachidonic acid metabolites in homeostasis: non-steroidal anti-inflammatory drugs, renal function and biochemical, histological and clinical effects and drug interactions. Drugs 33 [Suppl 1]: 56–66
Reyes JL, Meléndez E (1990) Effects of eicosanoids on the water and sodium balance of the neonate. Pediatr Nephrol 4: 630–634
Blackshear JL, Davidman M, Stillman MT (1983) Identification of risks for renal insufficiency from non steroidal anti-inflammatory drugs. Arch Interm Med 143: 1130–1134
Winther JB, Hoskins E, Printz MP, Mendoza SA, Kirkpatrick SE, Friedman WF (1980) Influence of indomethacin on renal function in concious newborn lambs. Biol Neonate 38: 76–84
Catterton Z, Sellers B, Gray B (1980) Inulin clearance in the premature infant receiving indomethacin. J Pediatr 96: 737–739
Witter FR, Niebyl JR (1986) Inhibition of arachidonic acid metabolism in the perinatal period: pharmacology, clinical application and potential adverse effects. Semin Perinatol 10: 316–333
Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE (1976) Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med 295: 526–529
Von Stockhausen V, Seyberth HW (1990) Effect of highly overdosed indomethacin in a preterm infant with symptomatic patent ductus arteriosus. Eur J Pediatr 149: 651–653
Taub M, Sato G (1980) Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol 105: 369–378
Rodriguez MG, Reyes JL (1992) Prostaglandin E2 modifies the intracellular pH in cultured renal cells (MDCK) (abstract) Proceedings of the 8th International Conference on Prostaglandins and Related Compounds, Montreal, 26–31 July, Montreal, Canada, p. 76
Novy MJ (1978) Effects of indomethacin on labor, fetal oxygenation and fetal development in rhesus monkeys. Adv Prostaglandin Thromboxane Res 4: 285–300
Hahne B, Selen G, Persson AEG (1984) Indomethacin inhibits renal functional adaptation to nephron loss. Renal Physiol 7: 13–21
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reyes, J.L. Arachidonic acid metabolites and haemodynamics of the neonate. Pediatr Nephrol 7, 841–844 (1993). https://doi.org/10.1007/BF01213371
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01213371