Summary
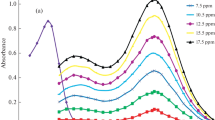
PPP forms an orange-red coloured complex with rhodium(III) at room temperature (27°) in the presence of sodium acetate-hydrochloric acid buffer of pH 1.0–3.0 containing copper(II) and ascorbic acid. The complex exhibits absorption maximum at 470 nm. Beer's law is valid over the rhodium concentration range 0.1–18μg/ml. Sandell's sensitivity of the reaction is 1.8·10−3 μg Rh/cm2 and the molar extinction coefficient is 5.68×103 l·mol−1cm−1 at 470 nm. The composition of the complex is 1∶1 and the apparent stability constant of the complex at pH 2.5 and 27° has the logK value of 4.0. The proposed method has been used for the determination of rhodium in thermocouple wires and in synthetic mixtures containing palladium, ruthenium, osmium, uranium or iridium.
Zusammenfassung
PPP bildet mit Rh(III) bei Zimmertemperatur (27°) in Gegenwart von Natriumacetat-Salzsäure (pH 1,0–3,0), Cu(II) und Ascorbinsäure eine orange-rote Komplexverbindung mit einem Absorptionsmaximum bei 470 nm. Das Beersche Gesetz gilt für Konzentrationen von 0,1 bis 18μg/ml. Die Empfindlichkeit nach Sandell beträgt 1,8×10−3 μg Rh/cm2; der molare Extinktionskoeffizient bei 470 nm ist 5,68×103 l·mol−1·cm−1. Die Zusammensetzung der Komplexverbindung entspricht dem Verhältnis 1∶1, die scheinbare Stabilitätskonstante bei pH 2,5 und 27° entspricht log K=4,0. Das vorgeschlagene Verfahren diente zur Rh-Bestimmung in Thermoelementdraht sowie in synthetischen Gemischen aus Pd, Ru, Os, U und Ir.
Similar content being viewed by others
References
F. E. Beamish, The Analytical Chemistry of the Noble Metals, Oxford: Pergamon. 1966. pp. 77, 79, 271, 409.
H. S. Gowda and B. Keshavan, Mikrochim. Acta [Wien]1975 I, 437;1977 I, 211.
H. S. Gowda and B. Keshavan, Indian J. Chem.15 A, 762 (1977).
B. Keshavan and P. Nagaraja, Microchem. J.30, 175 (1984).
B. Keshavan and P. Nagaraja, Indian J. Chem.22 A, 725 (1983).
B. Keshavan, Analyst106, 461 (1981).
F. E. Beamish and J. C. Van Loon, The Analytical Chemistry of the Noble Metals, Oxford: Pergamon. 1972. p. 34.
T. S. Ma and R. C. Rittner, Modern Organic Elemental Analysis. New York: Dekker. 1979.
G. H. Ayres, Analyt. Chemistry21, 652 (1949).
P. Job, Ann. chim.9, 113 (1928).
H. Irving and T. B. Pierce, J. Chem. Soc.1959, 2565.
J. H. Yoe and A. L. Jones, Ind. Eng. Chem., Analyt. Ed.16, 111 (1944).
R. T. Foley and R. C. Anderson, J. Amer. Chem. Soc.70, 1195 (1948);71, 909 (1949).
A. K. Mukherji and A. K. Dey, Analyt. Chim. Acta18, 324 (1958); J. Inorg. Nucl. Chem.6, 314 (1958).
K. Fasihuddin, S. K. Sindhwani, and R. P. Singh, Rev. Roum. chim.25, 875 (1980).
A. K. Singh, K. Mohan, and R. P. Singh, Indian J. Chem.19 A, 712 (1980).
Y. I. Usatenko, V. V. Velichko, V. K. Kudinova, and N. S. Stepanova, Ukr. Khim. Zh.46, 858 (1980).
H. Kulshreshtha, R. B. Singh, and R. P. Singh, Chem. Ind.17, 699 (1980).
B. K. Deshmukh and R. B. Karat, Fert. Tech.17, 202 (1980).
R. B. Singh, Y. P. S. Tomar, P. Jain, and B. S. Garg, J. Indian Inst. Sci.63B, 21 (1981).
R. M. Uttarwar and A. P. Joshi, J. Indian Chem. Soc.58, 898 (1981).
A. Muellorova and L. Geramakova, Chem. Zvesti.35, 651 (1981).
V. M. Savostina, O. A. Shpigun, and T. V. Chebrikova, Zh. Analyt. Khim.37, 285 (1982).
R. Gerhard, P. Bernd, and S. Ruediger, Z. Chem.22, 338 (1982).
A. K. Singh, B. Roy, D. Nath, and R. P. Singh, Indian J. Chem.21A, 549 (1982).
R. B. Singh, Y. P. S. Tomar, V. Mehra, and P. Jain, J. Inst. Chem.54, 203 (1982).
B. Morelli, Analyst108, 959 (1983).
S. Jaya, T. P. Rao, and T. V. Ramakrishna, Analyst108, 1151 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Keshavan, B., Nagaraja, P. Propionyl promazine phosphate as a new reagent for the spectrophotometric determination of rhodium(III). Mikrochim Acta 84, 283–294 (1984). https://doi.org/10.1007/BF01212393
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01212393