Abstract
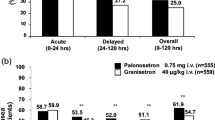
The efficacy and safety of a novel antiemetic, granisetron, was assessed at two dose levels (40 μg/kg and 160 μg/kg) in a randomized, double-blind study of 504 patients undergoing treatment with a range of standard cytostatic therapies. In the first 24 h, 75% of patients in the lower-dose group and 81% in the higher were complete responders (i.e. experienced no vomiting and no, or only mild, nausea). Two additional doses of granisetron (40 μg/kg) were allowed on the first day to treat any symptoms of nausea and vomiting. This produced improvement or resolution of symptoms in 94% of patients in the lower-dose group and 97% of patients in the higher-dose group. Over the 7 days of the study a complete response was maintained by 56% of patients in each group. No differences in efficacy or safety between the two doses of granisetron were established. Granisetron was very well tolerated. The commonest adverse event was headache, occurring in about 15% of patients, which required no more than simple analgesia. No extrapyramidal effects were observed. There was no relationship between the total dose of granisetron and the number or severity of specific adverse events.
Similar content being viewed by others
References
Bermudez J, Boyle EA, Miner WD, Sanger GJ (1988) The antiemetic potential of the 5-hydroxytryptamine-3 receptor antagonist BRL 43694A. Br J Cancer 58:644–650
Blower PR (1990) The role of specific 5-HT3 receptor antagonist in the control of cytostatic drug-induced emesis. Eur J Cancer 26 [Suppl 1]:S8-S11
Boyle EA, Miner WD, Sanger GJ (1987) Different anticancer therapies evoke emesis by mechanism that can be blocked by the 5HT3 receptor antagonist BRL 43694A. Br J Pharmacol 91:418P
Chevallier B on behalf of the Granisetron Study Group (1990) Efficacy and safety of granisetron compared with high dose metoclopramide plus dexamethasone in patients receiving high dose cisplatin in a single-blind study. Eur J Cancer 26 [Suppl 1]:S33-S36
Cupissol DR, Serrou B, Cabel M (1990) The efficacy of granisetron as a prophylactic antiemetic and interventional agent in high dose cisplatin-induced emesis. J Cancer Clin Oncol 26 [Suppl 1]:S23–27
Folb PI (1988) Cytostatic and immunosuppressive drugs. In: Dukes MNG (ed) Meyler's side effects of drugs. Elsevier, Amsterdam, pp 928–960
Ibrahim EM, Al-Idrissi HY, Ibrahim A (1988) Antiemetic efficacy of high dose dexamethosone: randomised, double-blind, crossover study with high dose metoclopramide in patients receiving cancer chemotherapy. Eur J Cancer Clin Oncol 22:283–288
Kamanabrou D on behalf of the Granisetron Study Group (1991) Intravenous granisetron: establishing the optimal dose (abstract). Abstract presented at ECCO6 Satellite Symposium, Florence, 1991
Kris MG, Tyson LB, Gralla RJ (1983) Extrapyramidal reaction with high dose metoclopramide. N Engl J Med 309:433–434
Laszlo J (1983) Emesis as limiting toxicity in cancer chemotherapy. In: Laszlo J (ed) Antiemetics and cancer chemotherapy. Williams & Williams, Baltimore, pp 1–5
Marty M on behalf of the Granisetron Study Group (1990) A comparative study of the use of granisetron, a selective 5HT3 antagonist, versus a standard antiemetic regimen of chlorpromazine plus dexamethasone in the treatment of cytostatic-induced emesis. Eur J Cancer 26 [Suppl 1]:528–532
Miner WD, Sanger GJ (1986) Inhibition of cisplatin induced vomiting by selective 5-hydroxytryptamine M receptor antagonism. Br J Pharmacol 88:497–499
Roila F, Tonato M, Basurto C (1985) Antiemetic activity of two different high doses of metoclopramide in cisplatin treated patients. Cancer Treat Rep 69:1353–1357
Upward JW, Arnold BDC, Link C, Pierce DM, Allen A, Tasker TCG (1990) The clinical pharmacology of granisetron (BRL 43694), a novel specific 5-HT3 antagonist. Eur J Cancer 26 [Suppl 1]:S12-S14
Author information
Authors and Affiliations
Consortia
Additional information
The Granisetron Study Group comprises: Dr. B. N. Bui, Fondation Bergonie, Bordeaux, France; Prof. G. Falkson, HF Verwoerd Hospital and University of Pretoria, Pretoria, South Africa; Dr. E. A. Hacking, Groote Schuur Hospital, Cape Town, South Africa; Dr. H. Bleiberg, Institut Jules Bordet, Brussels, Belgium; Dr. D. Cupissol, Hopital Val D'Aurelle II, Montpellier, France; Prof. F. Oberling, Hospital de Haute-Pierre, Strasbourg, France; Prof. G. H. Blijham, University Hospital, Utrecht, The Netherlands; Dr. M. Soukop, Glasgow Royal Infirmary, Glasgow, UK; Dr. J. F. Heron, Centre Francois Baclesse, Caen, France; Dr. A. Riviere, Centre Francois Baclesse, Caen, France; Dr. P. Fumoleau, Centre René Gauducheau, Nantes, France; Dr. I. E. Smith, The Royal Marsden Hospital, London, UK; Dr. B. Coiffier, Hopital Jules Courmont, Lyon, France; Dr. H. Roche, Centre Claudius Regaud, Toulouse, France; Dr. L. S. van Heukelom, Medisch Centrum Alkmaar, Alkmaar, The Netherlands; Dr. D. Cunningham, Ludwig Institute of Cancer Research, London, UK; Dr. P. Harper, Guy's Hospital, London, UK; Dr. J. W. R. Nortier, St Inrichting van Diakonessen, Utrecht, The Netherlands; Dr. L. Cals, Hopital Coste-Boyere, La Garde, Toulon, France; Prof. S. B. Kaye, Western Infirmary, Glasgow, UK; Dr. R. Favre, Institute J. Paoli, 1 Calmettes, Marseille, France; Prof. J. J. Sotto, C.H.R.U. de Grenoble, Grenoble, France; Prof. M. Schneider, Centre Antoine Lacassange, Nice, France; Dr. H. J. Keizer, Academisch Ziekenhuis, Leiden, The Netherlands; Dr. G. W. Allan, Stobhill Hospital, Glasgow, UK; Dr. M. C. O. Fehmers, St Samenwerkende Ziekenhuis de Stadsmaten, Enschede, The Netherlands; Prof. K. Brunner, Inselspital Bern, Bern, Switzerland; Dr. M. J. Leyland, East Birmingham Hospital, Birmingham, UK; Dr. A. H. Calvert, Institute of Cancer Research, Sutton, UK; Dr. C. Schop, Ziekenhuis Eudokia, Rotterdam, The Netherlands; Dr. P. H. T. J. Slee, St Antobnius Ziekenhuis, Nieuwegein, The Netherlands; Dr. C. S. Dott, SmithKline Beecham Pharmaceuticals, Reigate, UK.
Rights and permissions
About this article
Cite this article
Smith, I.E., Granisetron Study Group. A dose-finding study of granisetron, a novel antiemetic, in patients receiving cytostatic chemotherapy. J Cancer Res Clin Oncol 119, 350–354 (1993). https://doi.org/10.1007/BF01208844
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01208844