Abstract
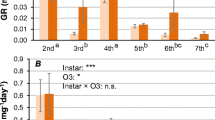
The effects of food plant on larval performance and midgut detoxification enzymes were investigated in larvae of the luna moth,Actias luna. Neonate larvae were fed leaves of black cherry, cottonwood, quaking aspen, white willow, red oak, white oak, tulip tree, paper birch, black walnut, butternut, or shagbark hickory. First instar survival, larval duration, and pupal weights were monitored as indices of food quality. Midgut enzyme preparations from fifth instars were assayed for β-glucosidase, quinone reductase, polysubstrate monooxygenase, esterase, and glutathione transferase activities. Larval survival on seven of the 11 plant species, including several recorded host plants, was extremely poor. Larvae performed well, and quite similarly, on birch, walnut, butternut, and hickory. Activities of all enzyme systems except β-glucosidase were significantly influenced by larval host plant. Of the systems assayed, quinone reductase and glutathione transferase activities were especially high. Comparisons of these values with published values for other Lepidoptera support the hypothesis that these enzyme systems are involved in conferring tolerance to juglone and related quinones occurring in members of the plant family Juglandaceae. Results suggest that host plant utilization by luna is more specialized at the individual or population level than at the species level and that biochemical detoxification systems may play a role in such specialization.
Similar content being viewed by others
References
Baker, W.L. 1972. Eastern Forest Insects. U.S. Department of Agriculture, Washington, D.C.
Brattsten, L.B. 1987a. Metabolic insecticide defenses in the boll weevil compared to those in a resistance-prone species.Pestic. Biochem. Physiol. 27:1–12.
Brattsten, L.B. 1987b. Inducibility of metabolic insecticide defenses in boll weevils and tobacco budworm caterpillars.Pestic. Biochem. Physiol. 27:13–23.
Brattsten, L.B., Price, S.L., andGunderson, C.A. 1980. Microsomal oxidases in midgut and fatbody tissues of a broadly herbivorous insect larva,Spodoptera eridania Cramer (Noctuidae).Comp. Biochem. Physiol. 66C:231–237.
Cohen, E. 1986. Glutathione-S-transferase activity and its induction in several strains ofTribolium castaneum.Entomol. Exp. Appl. 41:39–44.
Fox, L.R., andMorrow, P.A. 1981. Specialization: Species property or local phenomenon?Science 211:887–893.
Gilbert, B.L., Baker, J.E., andNorris, D.M. 1967. Juglone (5-hydroxy-1,4-naphthoquinone) fromCarya ovata, a deterrent to feeding byScolytus multistriatus.J. Insect Physiol. 13:1453–1459.
Gunderson, C.A., Brattsten, L.B., andFleming, J.T. 1986. Microsomal oxidase and glutathione transferase as factors influencing the effects of pulegone in southern and fall armyworm larvae.Pestic. Biochem. Physiol. 26:238–249.
Hansen, L.G., andHodgson, E. 1971. Biochemical characteristics of insect microsomes.N- andO-demethylation.Biochem. Pharmacol. 20:1569–1578.
Hedin, P.A., Collum, D.H., Langhans, V.E., andGraves, C.H. 1980. Distribution of juglone and related compounds in pecan and their effect onFusicladium effusum.J. Agric. Food Chem. 28:340–342.
Holland, W.J. 1968. The Moth Book. Dover Publications, New York.
Kapin, M.A., andAhmad, S. 1980. Esterases in larval tissues of gypsy moth,Lymantria dispar (L.): Optimum assay conditions, quantification and characterization.Insect Biochem. 10:331–337.
Lindroth, R.L. 1988. Hydrolysis of phenolic glycosides by midgut β-glucosidases inPapilio glaucus subspecies.Insect Biochem. In press.
Lindroth, R.L. 1989. Host plant alteration of detoxication enzyme activity inPapilio glaucus glaucus.Entomol. Exp. Appl. 50:29–35.
Margoliash, E., andFrohwirt, N. 1959. Spectrum of horse-heart cytochromec.Biochem. J. 71:570–572.
Norris, D.M. 1986. Anti-feeding compounds, pp. 97–146,in G. Haug and H. Hoffman (eds.). Chemistry of Plant Protection. 1. Sterol Biosynthesis, Inhibitors and Anti-Feeding Compounds. Springer-Verlag, New York.
Schacterle, G.R., andPollack, R.L. 1973. A simplified method for quantitative assay of small amounts of protein in biologic material.Anal. Biochem. 51:654–655.
Scriber, J.M., andFeeny, P. 1979. Growth of herbivorous caterpillars in relation to feeding specialization and to the growth form of their food plants.Ecology 60:829–850.
Segel, I.H. 1976. Biochemical Calculations, 2nd ed. John Wiley & Sons, New York.
Terriere, L.C. 1984. Induction of detoxication enzymes in insects.Anna. Rev. Entomol. 29:71–88.
Tietz, H.M. 1972. An Index to the Described Life Histories, Early Stages and Hosts of the Macrolepidoptera of the Continental United States and Canada. A.C. Allyn, Sarasota, Florida.
Wadleigh, R.W., andYu, S.J. 1987. Glutathione transferase activity of fall armyworm larvae toward α, β-unsaturated carbonyl allelochemicals and its induction by allelochemicals.Insect Biochem. 17:759–764.
Wilkinson, L. 1987. SYSTAT: The System for Statistics. SYSTAT, Inc., Evanston, Illinois.
Yu, S.J. 1983. Induction of detoxifying enzymes by allelochemicals and host plants in fall armyworm.Pestic. Biochem. Physiol. 9:330–336.
Yu, S.J. 1986. Consequences of induction of foreign compound-metabolizing enzymes in insects, pp. 153–174,in L.B. Brattsten and S. Ahmad (eds.). Molecular Aspects of Insect-Plant Associations. Plenum Press, New York.
Yu, S.J. 1987a. Quinone reductase of phytophagous insects and its induction by allelochemicals.Comp. Biochem. Physiol. 87B:621–624.
Yu, S.J. 1987b. Biochemical defense capacity in the spined soldier bug (Podisus maculiventris) and its lepidopterous prey.Pestic. Biochem. Physiol. 28:216–223.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindroth, R.L. Chemical ecology of the luna moth. J Chem Ecol 15, 2019–2029 (1989). https://doi.org/10.1007/BF01207434
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01207434