Abstract
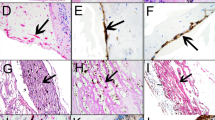
Proliferative vitreo-retinopathy (PVR) and subretinal membrane proliferation are the most common complication and cause of failure in retinal-detachment (RD) surgery. In this study, material withdrawn from 21 patients was observed. The vitreal taps of 16 bulbs affected by PVR and which had undergone vitrectomy, along with 5 bulbs obtained by enucleation, were stained with Hematoxylin Eosin and studied immunohistochemically. The cells involved in this proliferative tissue include macrophages, cellular elements of pigmented epithelium origin, fibroblasts and myofibroblasts. From the examination of enucleated bulbs, we can easily recognize that the cellular components of the membrane are represented by fibroblasts, capillaries, and occasional macrophages; meanwhile, PE cells remain at the base of the newly formed tissue.
Similar content being viewed by others
Abbreviations
- HE:
-
Hematoxylin-Eosin
- PE:
-
pigmented epithelium
- PVR:
-
proliferative vitreo-retinopathy
- RD:
-
retinal detachment
References
Duke-Elder SS. Disease of the retina, Vol. 10. London: Henry Kimpton, 1967, 771 pp.
Machemer R. Vitrectomy: A pars plana approach. New York: Grune E. Stratton, 1975.
Toti P, Morocutti A, Sforzi C, DeSanti MM, Catella AM, Baiocchi S. The subretinal fluid in retinal detachment: A cytologic study. Doc Ophthalmol 1991; 77: 39–46
Mackenzie HF, Tolentino FI. Proliferative vitreo-retinopathy (PVR). New York: Springer Verlag, 1988, 197 pp.
Mason DY. Immunocytochemical labelling of monclonal antibodies by the APAAP immunoalkaline phosphatase technique. Tech Immunocytochem 1985; 3: 25–42
Van Horn DL, Aaberg TM, Machemer R, Fenzl R. Glial cell proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol 1977; 84: 383–393.
Ussman JH, Lazarides E, Ryan JS. Traction retinal detachment. Arch Ophthalmol 1981; 99: 869–872.
Verdoon C, Renardel de Lavalette VW, Dalma-Weizhausz J, Orr GM, Sorgente N, Ryan SJ. Cellular migration, proliferation and contraction: An in vitro approach to a clinical problem-proliferative vitreoretinopathy. Arch Ophthalmol 1986; 104: 1216–1219.
Weller M, Heimann K, Wiedemann P. Demonstration of mononuclear phagocytes in a human epiretinal membrane using a monoclonal anti-human macrophage antibody. Graefe's Arch Clin Exp Ophthalmol 1988; 226: 252–254.
Weidemann P, Weller M. The pathophysiology of proliferative vitreoretinopathy. Acta Ophthalmol 1988, suppl 189; 66: 7–14.
Greco G, Bonanni R, Toti P, DiSanto A, Petracci M, Pannini S. Proliferative vitreo-retinopathy (PVR): An account of its pathogenesis. 9th International Congress of Eye Research, Helsinki, Finland, 29 July – 4 August 1990. Proceedings of the International Society for Eye Research 1990, Vol 6, 120 pp.
Yamomoto L, Yamashita H, Hori S. Electron microscopic observation of preretinal membranes. Jpn J Ophthalmol 1989; 33: 161–165.
Diegelmann F, Cohen IK, Kaplan AM. The role of macrophages in wound repair: A review. Plastic and Reconstructive Surgery 1981; 68: 107–113.
Fastenberg DM, Kenneth RD, Dorey K, Ryan SJ. The role of cellular proliferation in an experimental model of massive periretinal proliferation. Am J Ophthalmol 1982; 93: 56–572.
Kirchohof B, Kirchohof E, Ryan SJ, Dixon JFP, Barton BE, Sorgente N. Macrophage modulation of retinal pigment epithelial cell migration and proliferation. Greafe's Arch Clin Exp Ophthalmol 1989; 227: 60–66.
Weller M, Heiman K, Wiedemann P. Demonstration of mononuclear phagocytes in a human epiretinal membrane using a monoclonal anti-human macrophage antibody. Graefe's Arch Clin Exp Ophthalmol 1988; 11: 243–247.
Weller M, Wiedemann P, Heimann K, Zilles K. The significance of fibronectin in vitre-oretinal pathology; A critical evaluation. Greafe's Arch Clin Exp Opthalmol 1988; 226: 294–298.
Polverini PJ, Cotran RS, Gimbrone MA Jr, Unanue ER. Activated macrophages induce vascular proliferation. Nature 1977; 269: 208–806.
Martin BM, Gimbrone MA, Unanue ER, Cotran RS. Stimulation of nonlynphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol 1981; 126: 110–1515.
Shimokado K, Raines EW, Madtes DK, Barret TB, Benditt EP, Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell 198; 43: 277–286.
Cherfean GM, Smiddy WE, Michels RG, de La Cruz Z, Wilkinson CP, Green WR. Clinicopathologic correlation of pigmented epiretinal membranes. Am J Ophthalmol 1988; 106: 536–545.
Bitterman PB, Wewers MD, Adelberg RS, Crystal RS. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clinical lnvest 1986; 77: 700–708.
Ross R, Everett NB, Tyler R. Wound healing and collagen formation, 6: the origin of the wound fibroblast studied in parabiosis. J Cell Biol 1970; 44: 645–654.
Leibovich SJ, Ross R. A macrophages-dependent factor that stimulates the proliferation of fibroblast in vitro. Am J Pathol 1976; 84: 510–513.
Miller B, Miller H, Patterson R, Ryan SJ. Retinal wound healing: Cellular activity at the vitreoretinal interface. Arch Ophthalmol 1986; 104: 281–285.
Newsome DA, Rodrigues MM, Machemer R. Human massive periretinal proliferation: In vitro characteristics of cellular components. Arch Ophthalmol 1981; 99: 873–880.
Phan SH, Kunkel SL. Inhibition of Bleomycin-induced pulmonary fibrosis by nordihy-droguiaretic acid: The role of alveolar macrophage activation and mediator production. Am J Pathol 1986; 124: 343–352.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toti, P., Greco, G. & Catella, A.M. Morphological and pathogenetic aspects of proliferative vitreo-retinopathy. Doc Ophthalmol 88, 105–112 (1994). https://doi.org/10.1007/BF01204608
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01204608