Abstract
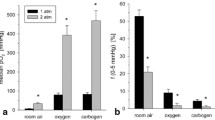
Tumor oxygen tensions were measured using a computer-controlledPO2 microelectrode in two preclinical solid tumor models, the rat 9L gliosarcoma and the rat 13672 mammary carcinoma. Tumor oxygenation profiles were determined under four conditions: (a) during normal air breathing, (b) during carbogen breathing, (c) after intravenous administration of a solution of ultrapurified polymerized bovine hemoglobin with normal air breathing and (d) after intravenous administration of a solution of ultrapurified polymerized bovine hemoglobin with carbogen breathing. Both tumors had severely hypoxic regions under normal air-breathing conditions. Although carbogen breathing increased the oxygenation of the better-oxygenated portions of the tumor, it made no impact on the severely hypoxic tumor regions. Administration of the hemoglobin solution was effective in increasing the oxygenation throughout both tumors under normal air-breathing conditions. The addition of carbogen breathing to administration of the hemoglobin solution eliminated severe hypoxia in the 9L gliosarcoma and markedly reduced the severely hypoxic regions of the 13672 mammary carcinoma. At 24 h after administration of the hemoglobin solution the 13672 mammary carcinoma showed greater hypoxia than before treatment, which was partially corrected with carbogen breathing.
Similar content being viewed by others
References
Adams GE (1990) The clinical relevance of tumour hypoxia. Eur J Cancer 26:420–421
Breepel PM, Kreuzer F, Hazevoet M (1981) Interactions of organic phosphates with bovine hemoglobin. I. Oxylabile and phosphatelabile proton binding. Pflügers Arch 389:219
Bunn HF (1971) Differences in the interaction of 2,3-diphosphoglycerate with certain mammalian hemoglobins. Science 172:1049–1050
Chang TMS (1988a) Attempts to find a method to prepare artificial hemoglobin corpuscles. Biomater Artif Cells Artif Organs 16:1–11
Chang TMS (1988b) Red blood cell substitutes: microencapsulated hemoglobin and cross-linked hemoglobin including pyridoxylated polyhemoglobin and conjugated hemoglobin. Biomater Artif Cells Artif Organs 16:11–29
Chang TMS (1992) Blood substitutes based on modified hemoglobin prepared by encapsulation or crosslinking: an overview. Biomater Artif Cells Immob Biotechnol 20:159–179
Cochin A, Das Gupta TK, DeWoskin R, Moss GS (1974) Immunologic properties of stroma vs. stroma-free hemoglobin solution. J Surg Oncol 4:19
Coleman CN (1988) Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. JNCI 80:310–317
Cunnington PG, Jenkins SN, Tam SC, Wong JT (1981) Oxygen-binding and immunological properties of complexes between dextran and animal hemoglobins. Biochem J 193:261–266
De Venuto F (1988a) Evaluation of human and bovine modified-hemoglobin solution as oxygen carrying fluid for blood volume replacement. Biomater Artif Cells Artif Organs 16:77–84
De Venuto F (1988b) Evaluation of human and bovine modified-hemoglobin solution as oxygen-carrying fluid for blood volume replacement. Biomater Artif Cells Artif Organs 16:77–83
Duncan W (1973) Exploitation of the oxygen enhancement ratio in clinical practice. Br Med Bull 29:33
Evans RG, Kimler BF, Morantz RA, Tribhawan SV, Gemer LS, O'Kell V, Low N (1989) A phase I–II study of the use of Fluosol-DA 20% as an adjuvant to radiation therapy in the treatment of primary high-grade brain tumors. Int J Radiat Oncol Biol Phys 19:415–420
Fowler JR, Thomlinson RH, Howes AE (1970) Time-dose relationships in radiotherapy. Eur J Cancer 6:207
Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH, Broder GJ (1988) Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 14:831–838
Herman TS, Teicher BA, Coleman CN (1990) The interaction of SR-4233 with hyperthermia and radiation in the FSaIIC murine fibrosarcoma tumor system in vitro and in vivo. Cancer Res 50:5055–5059
Hodakowski GT, Page RD, Harringer W, Jacobs EE Jr, La Raja PJ, Svizzero T, Guerrero JL, Austen WG, Vlahakes GJ (1992) Ultrapure polymerized bovine hemoglobin blood substitute: effects on the coronary circulation. Biomater Artif Cells Immob Biotechnol 20:669–672
Holden SA, Herman TS, Teicher BA (1990) Addition of a hypoxic cell selective cytotoxic agent (mitomycin C or porfiromycin) to treatment with Fluosol-DA/carbogen/radiation. Radiother Oncol 18:59–70
Kallinowski F, Zander R, Hoeckel M, Vaupel P (1990) Tumor tissue oxygenation as evaluation by computerized-pO2-histography. Int J Radiat Oncol Biol Phys 19:953–961
Keipert PE, Gomez CL, Gonzales A, Macdonald VW, Winslow RM (1992) The role of the kidneys in the excretion of chemically modified hemoglobins. Biomater Artif Cells Immob Biotechnol 20:737–745
Kim HW, Clancy T, Chen F, Greenburg AG (1992) Hepatic reticuloendothelial function following resuscitation with hemoglobin solutions. Biomater Artif Cells Immob Biotechnol 20:789–791
Laver MD, Jackson E, Scherperel M, Tung C, Radford EP (1977) Hemoglobin-O2 affinity regulation; DPF, monovalent ions, and hemoglobin concentration. J Appl Physiol 43:632
Lustig R, McIntosh-Lowe NL, Rose C, Haas J, Krasnow S, Spaulding M, Prosnitz L (1989) Phase I–II study of Fluosol-DA and 100% oxygen breathing as an adjuvant to radiation in the treatment of advanced squamous cell tumors of the head and neck. Int J Radiat Oncol Biol Phys 16:1587–1594
Lustig R, Lowe N, Prosnitz L, Spaulding M, Cohen M, Stitt S, Brannon R (1990) Phase I/II study of fluosol and 100% oxygen breathing as an adjuvant to radiation in the treatment of unresectable non small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 19:97–102
Martin DF, Porter EA, Fischer JJ, Rockwell S (1987a) Effect of a perfluorochemical emulsion on the radiation response of BA 1112 rhabdomysarcomas. Radiat Res 112:45–53
Martin DF, Porter EA, Rockwell S, Fischer JJ (1987b) Enhancement of tumor radiation response by the combination of a perfluorochemical emulsion and hyperbaric oxygen. Int J Radiat Oncol Biol Phys 13:747–751
Okunieff P, Dunphy EP, Hoeckel M, Terris DJ, Vaupel P (1992) The role of oxygen tension distribution on the radiation response of human breast carcinoma. Adv Exp Med Biol (in press)
Rapoport S, Guest GM (1941) Distribution of acid-soluble phosphorous in the blook cells of various vertebrates. J Biol Chem 138:269–280
Rooney MW, Hirsch LJ, Aasen MK, Goldberg SA, Mathru M (1992) Lack of increased cardiac output during hemoglobin hemodilution can be reversed with sodium nitroprusside. Biomater Artif Cells Immob Biotechnol 20:689–692
Rose CM, Lustig R, McIntosh N, Teicher BA (1986) A clinical trial of Fluosol-DA 20% in advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 12:1325–1327
Sartorelli AC (1988) Therapeutic attack of hypoxic cells of solid tumors: preclinical addess. Cancer Res 48:775–778
Siemann DW (1992) Keynote address: tissue oxygen manipulation and tumor blood flow. Int J Radiat Oncol Biol Phys 22:393–395
Teicher BA, Lazo JS, Sartorelli AC (1981) Classification of antineoplastic agents by their selective toxicities toward oxygenate and hypoxic tumor cells. Cancer Res 41:73–81
Teicher BA, Holden SA, Rose CM (1985a) Differential enhancement of melphalan cytotoxicity in tumor and normal tissue by Fluosol-DA and oxygen breathing. Int J Cancer 36:585–589
Teicher BA, Bernal SD, Holden SA, Cathcart KNS (1988a) Effect of Fluosol-DA/carbogen on etoposide/alkylating agent antitumor activity. Cancer Chemother Pharmacol 21:281–285
Teicher BA, Herman TS, Holden SA, Cathcart KNS (1988b) The effect of Fluosol-DA and oxygenation status on the activity of cyclophosphamide in vivo. Cancer Chemother Pharmacol 21:286–291
Teicher BA, Holden SA, Cathcart KNS, Herman TS (1988c) Effect of various oxygenation conditions and Fluosol-DA on the cytotoxicity and antitumor activity of bleomycin. JNCI 80:599–603
Teicher BA, Herman TS, Rose CM (1988d) Effect of fluosol-DA on the response of intracranial 9L tumors to x rays and BCNU. Int J Radiat Oncol Biol Phys 15:1187–1192
Teicher BA, Holden SA, Crawford JM (1988e) Effects of Fluosol-DA and oxygen breathing on adriamycin antitumor activity and cardiac toxicity in mice. Cancer 61:2196–2201
Teicher BA, McIntosh-Lowe NL, Rose CM (1988f) Effect of various oxygenation conditions and Fluosol-DA on cancer chemotherapeutic agents. Biomater Artif Cells Artif Organs 16:533–546
Teicher BA, Herman TS, Jones SM (1989) Optimization of perfluorochemical levels with radiation therapy in mice. Cancer Res 49:2693–2697
Teicher BA, Holden SA, Al-Achi A, Herman TS (1990) Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res 50:3339–3344
Teicher BA, Herman TS, Hopkins RE, Menon K (1992a) Effect of a bovine hemoglobin preparation on the response of the FSaIIC fibrosarcoma to chemotherapeutic alkylating agents. J Cancer Res Clin Oncol 118:123–128
Teicher BA, Holden SA, Ara G, Herman TS, Hopkins RE, Menon K (1992b) Effect of a bovine hemoglobin preparation (SBHS) on the response of two murine solid tumors to radiation therapy or chemotherapeutic alkylating agents. Biomater Artif Cells Immob Biotechnol 20:657–660
Teicher BA, Holden SA, Ara G, Sotomayor EA, Menon K, Tarbell NJ, Sallan SE (1992c) Etanidazole as a modulator of combined modality therapy in the rat 9L gliosarcoma. Int J Oncol 1:625–630
Teicher BA, Herman TS, Frei E III (1992d) Perfluorochemical emulsions: oxygen breathing in radiation sensitization and chemotherapy modulation. In: Devita VT Jr, Hellman S, Rosenberg SA (eds) Important advances in oncology 1992. Lippincott, Philadelphia, pp 39–59
Teicher BA, Holden SA, Northey D, Dewhirst MW, Herman TS (1993) Therapeutic effect of infused Fluosol-DA/carbogen with ephendrine, flunarizine or nitroprusside. Int J Radiat Oncol Biol Phys (in press)
Vaupel P (1990) Oxygenation of human tumors. Strahlenther Onkol 166:377–386
Vaupel PW (1993) Oxygenation of solid tumors. In: Teicher BA (ed) Drug resistance in oncology. Dekker, New York, pp 53–85
Vaupel P, Schlenger K, Knoop C, Hockel M (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancer by computerized O2 tension measurements. Cancer Res 51:3316–3322
Weissberg JB, Son YH, Papac RJ, Sasaki C, Fischer DB, Lawerence R, Rockwell S, Sartorelli AC, Fischer JJ (1989) Randomized clinical trial of mitomycin C as an adjunct to radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Phys 17:3–9
Winslow RM (1988) Optimal hematologic variables for oxygen transport including P50, hemoglobin cooperativity, hematocrit, acid-base status, and cardiac function. Biomater Artif Cells Artif Organs 16:149–172
Author information
Authors and Affiliations
Additional information
This work is supported by NIH grant P01-CA19589 and a gift from Biopure Corporation, Boston, Mass.
Rights and permissions
About this article
Cite this article
Teicher, B.A., Schwartz, G.N., Sotomayor, E.A. et al. Oxygenation of tumors by a hemoglobin solution. J Cancer Res Clin Oncol 120, 85–90 (1993). https://doi.org/10.1007/BF01200729
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01200729