Abstract
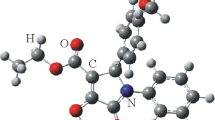
The crystal and molecular structure of methyl 1-phenyl-3,4-dioxo-2-naphthalenecarboxylate, C18H12O4, has been determined from three-dimensional, single-crystal X-ray diffraction data. The compound crystallizes in the monoclinic space groupCc(No. 9, C 4s ) witha=9.837(2),b=16.397(3),c=8.706(1) Å,β=94.88(1)°,Z=4,D m =1.38(1) Mg m−3, andD x =1.388 Mg m−3. The phasing model was determined by direct methods and the final full-matrix least-squares refinement yieldedR=0.0363 and Rw=0.0405 for 1374 unique reflections. Optical, infrared, NMR, and UV-VIS analyses have also been carried out. The molecules in the crystal lattice are held together by van der Waals forces.
Similar content being viewed by others
References
Bechtel, F., Chasseau, D., Gaultier, J., and Hauw, C. (1976)Acta Crystallogr. B 32, 1738.
Belew, J. S., and Layton, R. (1956) Presented at the 129th Meeting of the American Chemical Society, Dallas, TX. p. 31.
Bidics,Bond index of the Determination of Inorganic Crystal Structures, Institute for Material Research, Hamilton, Canada, 1969–1981.
Boeyens, J. C. A. (1976)J. Cryst. Mol. Struct. 6, 217.
Demitras, J. C., Russ, C. R., Salmon, G. F., Weber, J. H., and Weiss, G. S. (1972)Inorganic Chemistry (Prentice-Hall, Englewood Cliffs, N.J.), p. 35.
Goldschmidt, S., and Graef, R. (1928)Chem. Ber. 61, 1862.
International Tables for X-ray Crystallography (1974) Vol. IV (Kynoch Press, Birmingham, England).
Layton, R. (1956)Thesis. University of Virginia.
Macbeth, A. K., Price, J. P., and Winzor, F. L. (1935)J. Chem. Soc. 325.
Mullica, D. F., Korp, J. D., Milligan, W. O., Belew, J. S., McAtee, Jr. J. L., and Karban, J. (1979)J. Chem. Soc. Perkins Trans. II, 1703.
Mullica, D. F., Milligan, W. O., Belew, J. S., Grossie, D. A., and Sappenfield, E. L. (1984)Acta Crystallogr. C 40, 1923.
Pauling, L. (1960)The Nature of the Chemical Bond (third edition), (Cornell University Press, Ithaca, N.Y.), p. 257.
Rogers, D. (1981)Acta Crystallogr. A 37, 734.
Shelxtl-PC (1989) Siemens Analytical X-ray Instruments, Madison, WI.
Author information
Authors and Affiliations
Additional information
3-Carbomethoxy-4-phenyl-1,2-naphthoquinone.
Rights and permissions
About this article
Cite this article
Mullica, D.F., Belew, J.S., Hunt, S.K. et al. Spectroscopic and crystal structure analyses of methyl 1-phenyl-3,4-dioxo-2-naphthalenecarboxylate, C18H12O4 . Journal of Crystallographic and Spectroscopic Research 22, 303–308 (1992). https://doi.org/10.1007/BF01199532
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01199532