Summary
-
1.
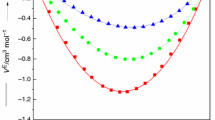
The specific viscosities have been determined of dilute solutions in benzene and in carbon tetrachloride of phosphorous and boric esters having long hydrocarbon radicals (from octyl to decyl. Comparison of the values found for ηsp (1.4%) and Zη with those calculated indicates that the molecules of these esters are in the extended form in dilute solution, which is in agreement with parachor data.
-
2.
The specific viscosities have been determined of dilute solutions in benzene and in carbon tetrachloride ] of phosphoric and phosphorotionic esters having hexyl and octyl radicals. Comparison of the values found for ηsp (1.4%) and Zη with those calculated indicates that the molecules of phosphoric and phosphorothionic esters are in the extended form in dilute solution which is not in agreement with conclusions based on a study of the parachors of these esters.
-
3.
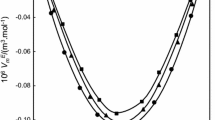
When a comparison is made of the found and calculated values of ηsp (1.4%) and Zη for macrocyclic compounds having rings of 15–34 members for which viscosity, data are available in the literature, it is found that the viscosity values found are closer to those calculated for the total number of members in the ring than to those calculated, following Staudinger's views, for a half of that number.
-
4.
Cyclic esters of triethylene glycol with succinic, adipic, and sebacic acids have been synthesized, and viscosity measurements have been made on their dilute solutions. The values found for ηsp (1,4%) and Zη are greater than those calculated for the total number of members in the ring,
-
5.
Our results show that further investigation is required into the question of what measurements of viscosity tell us concerning molecules of branched structure and molecules having branched rings, and they show that further investigation. is required also into the nature of Gibling's corrections for parallelism in the determination of molecular structure by the parachor method.
Similar content being viewed by others
Literature cited
H. Staudinger, “Macromolecular Organic Compounds. Rubber and Cellulose”, United Sci. Tech. Press, 1935, p. 57; H. Staudinger and F. Staiger, Ber. 68, 707 (1935); H. Staudinger and H. Schwalenstocker, ibid, 727; H Staudinger and A. Steinhoper, ibid, 471, Ann. 517,56 (1935).
H. Staudinger and H. Schwalenstocker, Ber. 68, 728 (1935).
H. Staudinger and E. Ochiai, Z. phys. Chem. 158, 45 (1932).
H. Staudinger and K. Rossler, Ber. 69, 52 (1936).
H. Staudinger and H. Schwalenstocker, Ber. 68, 727 (1935).
Ibid.
Ibid.
V. Vinogradova, Sci. Mem. Kazan Univ. 110, Book 9, (1950).
B. A. Arbuzov and V. S. Vinogradova, Ibid., p. 21.
B. A. Arbuzov and V. S. Vinogradova, Proc. Acad. Sci. USSR, 55, 415 (1947).
B. A. Arbuzov and L. M. Guzhavina, Proc. Acad. Sci. USSR, 61, 63 (1948).
B. A. Arbuzov and V. S, Vinogradova, Bull. Acad. Sci, USSR Div. Chem. Sci. 733 (1951).
H. Staudinger, “Macromolecular Organic Compounds”, United Sci.Tech, Press, 1935, p, 78
H. Staudinger and R. C. Bauer, Helv. Chim. Acta. 17, 863 (1934).
H. Staudinger, Makromol. Chem. 4, 289 (1950).
H. Staudinger and Rossler, Ber. 69, 63 (1936).
H. Staudinger and Steiger, Ber. 68, 707 (1935).
B. A. Arbuzov and V. S. Vinogradova, Bull. Acad. Sci. USSR, Div. Chem. Sci, No. 3, 505 (1952).
T. Milobendzky, A. Sachnowsky, Ch. Polsky, 15, 34 (1917).
W. Carothers, J. Am. Chem. Soc. 57, 931 (1935).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arbuzov, B.A., Vinogradova, V.S. Viscosity-structure relationship for phosphorous, phosphoric, phosphorothionic, and boric esters. Russ Chem Bull 1, 773–780 (1952). https://doi.org/10.1007/BF01198863
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01198863