Summary
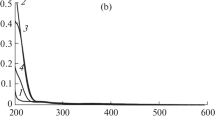
It has been established that 1-(2-phenyl-2-hydroxyiminoethyl)-1-quinolinium chloride, 1-(2-phenyl-2-hydroxyiminoethyl)-1-isoqui-nolinium chloride, 1-(2-phenyl-2-hydroxyiminoethyl)-1-(4′-methyl)-quinolinium chloride and 1-(2-phenyl-2-hydroxyiminoethyl)-1-(6′-methyl)quinolinium chloride react with palladium(II) chloride in the pH range 3.3–7.1 and form yellow water-soluble 1∶1 complexes with maximum absorbance at 413 nm. The conditional stability constants of the complexes at the optimum pH of 6.5 are all about 104.7, and the molar absorptivities are in the range 2.2–2.6×103 l·l mole−1·cm−1 at pH 6.5 and 413 nm. Beer's law is obeyed up to 3–4×10−4 M oxime concentration, depending on the oxime determined.
Similar content being viewed by others
References
Z. Binenfeld and V. Vojvodić, Försvars Median10, 114 (1974).
P. Malatesta, G. Quaglia, and E. Floresta, Farmaco (Ed. Sci.)23, 757 (1968).
Z. Binenfeld, B. Bošković, D. Rakin, and M. Ćosić, Acta Pharm. Jugosl.21, 113 (1971).
A. Granov, M. Maksimović, and Z. Binenfeld, Naučno-Tehn. Pregled. No. 2,34, 34 (1984).
B. Sen, Anal. Chem.30, 881 (1959).
B. Sen, Anal. Chem.31, 791 (1959).
W. J. Holland and J. Božić, Anal. Chem.40, 433 (1968).
C. K. Bhaskare and S. G. Kawatkar, Anal. Chim. Acta73, 405 (1974).
R. B. Singh, B. S. Garg, and R. P. Singh, Talanta26, 425 (1979).
K. Karljiković, B. Stanković, A. Granov, and Z. Binenfeld, Acta Pharm. Jugosl.33, 209 (1983).
A. Vogel, Quantitative Inorganic Analysis, 3rd Ed. London: Longmans 1961. p. 445.
J. A. Coch-Frugoni, Gazz. Chim. Ital.87, 403 (1957).
P. Job, Ann. Chim. Phys.9, 113 (1928).
W. C. Vosburgh and G. R. Cooper, J. Am. Chem. Soc.63, 437 (1941).
J. Yoe and A. Jones, Ind. Eng. Chem., Anal. Ed.16, 111 (1944).
H. Bent and C. French, J. Am. Chem. Soc.63, 568 (1941).
L. Sommer, V. Kuban, and J. Havel, Spectrophotometric Studies of the Complexation in Solution, Tomus XI, Chemia 7, opus1, 25 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karljiković, K., Stanković, B. & Binenfeld, Z. Spectrophotometric behaviour of quinolinium oxime-palladium(II) complexes. Mikrochim Acta 86, 195–202 (1985). https://doi.org/10.1007/BF01197819
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01197819