Abstract
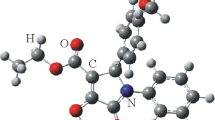
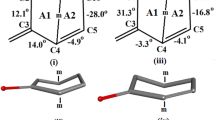
The crystal and molecular structure of the norditerpenoid alkaloid 1-epi-delphisine (5), C28H43NO8, M r 521.66, has been confirmed by as X-ray diffraction study using the SIR 88 analysis program. The alkaloid crystallizes in the space groupP21 with cell parameters:a=11.853(1)Å,b=10.511(1)Å,c=11.854(1)Å,β=112.58(1),V=1363.61(0)Å3,Z=2,D calc=1.27 g/cm3, λ (CuKα)=1.54184 Å,μ (CuKα=7.2 cm−1,F(000)=564, temperature 23°C,R=0.052, for 2907 reflections. Ring A of 1-epi-delphisine exists in a chair conformation. By comparison, delphisine which bears a C-1α hydroxyl group, has ring A in a boat conformation stabilized by an intramolecular N----H-O hydrogen bond. Ring D of both of these alkaloids exists in a boat form. Unambiguous proton and carbon-13 nmr assignments for delphisine, 1-epi-delphisine and delphinine have been made by a detailed analysis of the DEPT, COSY, HETCOR, COLOC, DIFNOE, and selective INEPT nmr techniques.
Similar content being viewed by others
References
Bax, A. (1984)J. Magn. Reson.,57, 314–318.
Bax, A., and Morris, L. (1981)J. Magn. Reson. 42, 501–505.
Bax, A., and Summers, M. F. (1986)J. Amer. Chem. Soc. 108, 2093–2094.
Burla, M. C., Camalli, M., Cascarano, G., Giacovazzo, G., Polidori, G., Spagna, R., and Viterbo, J. (1989)J. Appl. Crystallogr. 22, 398–393.
Cygler, M., Przybylska, M., and Edwards, O. E. (1982)Acta Crystallogr. B 35(5), 1500–1503.
Derome, A. E. (1987)Modern NMR techniques for chemistry research (Pergammon Press, New York) pp. 113–120.
Edwards, O. E., Marion, L., and Stewart, D. K. R. (1956)Can. J. Chem. 34, 1315–1328.
Edwards, O. E., and Przybylska, M. (1982)Can. J. Chem. 60, 2661–2667.
Joshi, B. S., and Pelletier, S. W. (1987)Heterocycles 26, 2503–2518.
Kessler, H., Griesinger, C., Zarbock, J., and Loosi, H. R. (1984)J. Magn. Reson. 57, 331–336.
MoIEN. (1990) (Ed)An interactive structure solution procedure, Enraf-Nonius (Delft, The Netherlands).
Pelletier, S. W., DeCamp, W. H., Lajsic, S., Djarmati, Z., and Kapadi, A. H. (1974)J. Amer. Chem. Soc. 96, 7815–7817.
Pelletier, S. W., and Djarmati, Z. J. (1976)Amer. Chem. Soc. 98, 2626–2636.
Pelletier, S. W., Djarmati, Z., Lajsic, S., and DeCamp, W. H. (1976)J. Amer. Chem. Soc. 26, 2617–2625.
Pelletier, S. W., and Etse, J. T. (1989)J. Nat. Prod. 52, 145–152.
Pelletier, S. W., Mody, N. V., Joshi, B. S., and Schramm, L. C., (1984) inAlkaloids: chemical and biological perspectives, Vol. 2, chapter 5, S. W. Pelletier (ed.) (Wiley, New York), pp. 205–462.
Pelletier, S. W., Mody, N. V., Varughese, K. L., Maddry, J. A., and Desai, H. K. (1981)J. Amer. Chem. Soc. 103, 6536–6538.
Przybylska, M. (1961)Acta Cryst. 14, 424–429.
Przybylska, M., and Marion, L. (1956)Can. L. Chem. 34, 185–187.
Przybylska, M., and Marion, L. (1959)Can. L. Chem. 37, 1843–1845.
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Dr. K. Krishna Bhandary (1946–1992).
Rights and permissions
About this article
Cite this article
Joshi, B.S., Desai, H.K., Bhandaru, S. et al. Crystal and molecular structure of 1-epi-delphisine and nmr assignments for delphisine, 1-epi-delphisine and delphinine. Journal of Crystallographic and Spectroscopic Research 23, 877–883 (1993). https://doi.org/10.1007/BF01195735
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01195735