Abstract
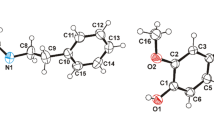
X-ray crystal structures of 4-amino-3-(2-thienyl) butyric acid (compound1), 4-amino-3-(4-bromo-2-thienyl) butyric acid (compound2), and 4-amino-3-(5-methyl-2-furyl) butyric acid (compound3) are reported. Space groups and unit/cell parameters are: compound1, monoclinic,P21 c,a=13.288(3),b=5.231(1),c=12.388(2)Å,β=92.3(1)°; compound2, monoclinic,P21/c,a=12.610(7),b=5.156(1),c=15.814(8)Å,β=101.8(1)°; compound3, orthorhombic, Pccn,a=11.461(1),b=25.284(2),c=6.977(1)Å. FinalR indices are: compound1, 0.057; compound2, 0.069; compound3, 0.060. Conformations of their γ-aminobutyric chains are compared with the one of γ-amino-β-(p-chlorophenyl)-butyric acid (baclofen, compound4). Two different types of conformations are observed, i.e., conformations (i) with folding (compound3) or (ii) without folding (compounds1,2, and4) of the ammonium group toward the heteroaromatic or aromatic ring. However, distances between ionized groups are constant.
Similar content being viewed by others
References
Berthelot, P., Vaccher, C., Flouquet, N., Debaert, M., Luyckx, M., and Brunet, C. (1991a)J. Med. Chem. 34, 2557.
Berthelot, P., Vaccher, C., Flouquet, N., Luyckx, M., Brunet, C., Boulanger, T., Frippiat, J. P., Vercauteren, D. P., Evrard, G., and Duranl, F. (1991b)Eur. J. Med. Chem. 26, 315.
Bowery, N. (1989)T.I.P.S. 10, 401.
Chang, C. H., Yang, D. S. C., Too, C. S., Wang, B-C., Pletcher, J., Sax, M., Terrence, C. F. (1982)Acta Crystallogr. B 38, 2065.
Coluci, W. J., Gandour, R. D., Mooberry, E. A. (1986)J. Am. Chem. Soc. 108, 7141.
Fourme, R. (1972)Acta Crystallogr. B 28, 2984.
Humblet, C., Durant, F., and Evrard, G. (1977)Acta Crystallogr.B 33, 1615.
Johnson, C. J. (1965)ORTEP. Report ORNL-3794. (Oak Ridge National Laboratory, Tenn.).
Madesclaire, M., Roche, D., and Carpy, A. (1988)C. R. Acad. Sci. Paris 306, 891.
Michel, A., Gustin, R., Evrard, G., and Durant, F. (1982a)Bull. Soc. Chim. Belg. 91, 49.
Michel, A., Gustin, R., Evrard, G., and Durant, F. (1982b)Bull. Soc. Chim. Belg. 91, 123.
Ogata, N. (1990)Gen. Pharmac. 21, 395.
Sheldrick, G. M. (1976),SHELX-76. Program for crystal structure determinations. (University of Cambridge, United Kingdom).
Sheldrick, G. M. (1986),SHELXS-76. Program for crystal structure determinations. (University of Göttingen, Germany).
Stewart, J. (1976) Editor XRAY-76 Tech. Rep., Computer Sciences Center, University of Maryland, U.S.A.
Streurman, H. J., and Schenk, H. (1970)Rec. Trav. Chim. Pays-Bas (Rec. J. R. Neth. C.) 89, 392.
Vaccher, C., Berthelot, P., Flouquet, N., Marko, J., Debaert, M. (1991)Spectrochim. Acta A 47, 1071.
Van der Brempt, C. (1986) Ph.D. Thesis, University of Namur, Belgium.
Weast, R. C. (1986–1987)Handbook of chemistry and physics. (CRC Press, Inc. Boca Raton, Florida). D-188.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pirard, B., Evrard, G., Norberg, B. et al. Crystal structures of baclofen analogs: 3-thienyl- and 3-furylaminobutyric acids. Journal of Crystallographic and Spectroscopic Research 23, 843–848 (1993). https://doi.org/10.1007/BF01195730
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01195730