Abstract
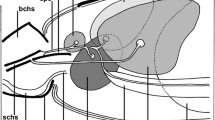
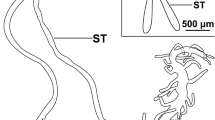
We describe the ultrastructure of type-I salivary-gland acini in two argasid and two ixodid species. The basic cell types in the agranular or type-I acini, and their associations, are very similar in argasids and ixodids; therefore, we propose an anatomical nomenclature for cells in the type-I acinus based on the adult ixodidsAmblyomma americanum andDermacentor variabilis, and the argasid adultArgas (Persicargas) arboreus and on nymphalOrnithodoros moubata. Four cell types were present in all specimens: one central lamellate cell, a variable number of peripheral lamellate cells, a variable number of peritubular cells depending on the species, and one circumlumenal cell. The lamellate cells had infolded basal plasma membranes that presented an amplified surface area to the hemolymph. These cells most likely secreted the fluid involved in water vapor uptake by ticks. ForAmblyomma americanum females, abundant K+-dependent, ouabain-sensitive Na+, K+-ATPase complexes were located on the infolded basal plasma membranes of the lamellate cells. Apical membranes of the lamellate cells, and plasma membranes of other cell types in the acinus had little or no evidence of Na+, K+-ATPase activity. Only the central lamellate cell extended from the hemolymph of the acinus to its lumen; peripheral cells did not contact the lumen. Except when the ticks were rehydrating, lipid inclusions were common features in the lamellate cells of the ixodids. Lipid inclusions were not seen in argasid type I acini; however, glycogen deposits were common. To determine if acinar cells respond to the changing hydration state of the tick, unfed femaleA. americanum were subjected to dehydration/rehydrating conditions. During rehydration, mitochondria in the lamellate cells changed from a matrix of medium electron-density and intermembrane space (orthodox configuration) to a matrix of greater density and larger intermembrane space (condensed configuration). The orthodox configuration was consistently observed in control and dehydrating ticks. The condensed configuration was the norm for mitochondria in lamellate cells of rehydrating ticks. Lipid inclusions were depleted in the rehydrating ticks compared to control or dehydrating ticks. Acini appeared to be reverting to the control or desiccated state when ticks were returned to low humidity, suggesting that these changes were cyclical. Nymphs ofO. moubata subjected to the same dehydration/rehydrating conditions showed no obvious ultrastructural changes.
Similar content being viewed by others
References
Balashov, Y.S., 1968. Bloodsucking ticks (Ixodoidea), vectors of diseases to man and animals. Akad. Nak SSSR Zool. Inst. Leningrad, 319 pp. (In Russion, 1967). (In English: Misc. Publ. Entomol. Soc. Am., (1972), 8: 161–376.
Balashov, Y.S., 1979. Atlas of the Electron Microscopic Anatomy of Ixodid Ticks, Nauka, Leningrad, 256 pp. (Eng. transl, Entomol. Soc. Am. Misc. Publ., 1983, 289 pp.)
Barker, D.M., Ownby, C.L., Krolak J.M., Claypool, P.L. and Sauer, J.R., 1984. The effects of attachment, feeding, and mating on the morphology of the type I alveolus of salivary glands of the lone star tick,Amblyomma americanum (L.). J. Parasitol., 70: 99–113.
Berridge, M.J. and Oschman, J.L., 1972. Transporting Epithelia. Academic Press, New York.
Binnington, K.C., 1978. Sequential changes in salivary gland structure during attachement and feeding of the cattle tick,Boophilus microplus. Int. J. Parasitol., 8: 97–115.
Candipan, R.C. and Sjostrand, F.S., 1984. An analysis of the contribution of the preparatory technique to the appearance of condensed and orthodox conformations of liver mitochondria. J. Ultrastruct. Res. 89: 281–294.
Chambers, V.C., 1973. The use of ruthenium red in an electron microscope study of cytophagocytosis. J. Cell Biol., 57: 874–878.
Coons, L.B., and Roshdy, M.A., 1973. Fine structure of the salivary glands of unfed maleDermacentor variabilis (Say) (Ixodoidea: Ixodidae). J. Parasitol. 59: 900–912.
Coons, L.B. and Roshdy, M.A., 1979. Functional morphology and cytochemical localization of chloride ions and ouabain sensitive phosphatase activity in the salivary gland transport epithelia of four species of ticks. In: J.G. Rodriquez, (Editor), Recent Advances in Acarology, Vol. I. Academic, New York, pp. 427–434.
Devine, T.L., 1982. Neutron activation analysis of saliva from the tickAmblyomma variegatum. Experientia, 38: 676–677.
Dzhafarov, T.E., 1965. An electron microscopy study of pyramidal alveoli cells of salivary glands of some ticks. Izd. Acad. Nauk. SSSR, Leningrad, pp. 31–37.
El Shoura, S.M., 1985. Ultrastructure of salivary glands ofOrnithodoros (Ornithodoros) moubata (Ixodoidea; Argasidae). J. Morphol., 186 45–52.
Frick, J.H. and Sauer, J.R., 1972. Examination of a biological cryostat/nanoliter osmometer for use in determining the freezing point of insect hemolymph. Ann. Entomol. Soc. Am., 66: 781–783.
Hackenbrock, C.R., 1966. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol., 30: 269–97.
Kahl, O., Hoff, R. and Knülle, W., 1990. Gross morphological changes in the salivary glands ofIxodes ricinus (Acari, Ixodidae) between bloodmeals in relation to active uptake of atmospheric water. Exp. Appl. Acarol., 9: 239: 258.
Kirkland, W.L., 1971. Ultrastructural changes in the nymphal salivary glands of the rabbit tick,Haemaphysalis leporispalustris during feeding. J. Insect Physiol., 17: 1933–1946.
Knülle, W. and Rudolph, D., 1982. Humidity relationships and water balance of ticks. In: F. Obenchain and R. Galun (Editors), Physiology of Ticks. Pergamon, New York, pp. 43–70.
Krolak, J.M., Ownby, C.L. and Sauer, J.R., 1982. Alveolar structure of salivary glands of the lone star tick.Amblyomma americanum (L.): Unfed females. J. Parasitol., 68: 61–82.
Mayahara, H., Fujimoto, K. and Ogawa, K., 1980. A new one-step method for the cytochemical localization of ouabain-sensitive, potassium-dependentp-nitrophenlyphosphatase activity. Histochemistry, 67: 125–138.
McGee-Russell, S.M. and Smale, N.B., 1963. On colouring epon-embedded tissue sections with Sudan black B or Nile blue A for light microscopy. O. J. Microsc. Sci., 104: 109–115.
McMullen, H.L., Sauer, J.R. and Burton, R.L., 1976. Possible role in uptake of water vapour by ixodid tick salivary glands. J. Insect Physiol., 22: 1281–1285.
Megaw, M.J.W. and Beadle, D.J., 1979. Structure and function of the salivary glands of the tick,Boophilus microplus Canestrini (Acarina: Ixodidae). Int. J. Insect Morphol. Embryol., 8: 67–83.
Meredith, J. and Kaufman, W.R., 1973. A proposed site of fluid secretion in the salivary gland of the ixodid tickDermacentor andersoni. Parasitology, 67: 205–217.
Mollenhauer, H.H., 1964. Plastic embedding mixtures for use in electron microscopy. Stain Technol., 39: 111–115.
Needham, G.R. and Coons, L.B., 1984. Ultrastructural changes in type I alveoli of salivary glands from hydrating and desiccating lone star ticks. In: D.E. Griffiths and C.E. Bowman (Editors), Acarology VI. Ellis Horwood, Chichester, pp. 336–373.
Needham, G.R. and Teel, P.D., 1986. Water balance by ticks between bloodmeals. In: J.R. Sauer and J.A. Hair (Editors), Morphology, Physiology and Behavioral Biology of Ticks. Ellis Horwood, Chichester, pp. 100–151.
Needham, G.R., Freda, T.J. and Coons, L.B., 1982. Cyclical ultrastructure changes in agranular salivary gland alveoli in response to humidity: a proposed model for ixodid tick rehydration. Am. Zool., 22: 898.
O'Donnell, M.J. and Machin, J., 1988. Water vapor absorption by terrestrial organisms. In: R. Gilles (Editor), Advances in Comparative and Environmental Physiology. Springer, Berlin, pp. 47–90.
Patrick, C.D. and Hair, J.A., 1975. Laboratory rearing procedures and equipment for multihost ticks (Acarina: Ixodidae). J. Med. Entomol., 12: 389–390.
Rosell-Davis, R., Coons, L. and Needham, G., 1985. Ultrastructural study of the salivary glands ofOrnithodoros moubata nymphs during feeding. Proc. Electron Microsc. Soc. Am., 43: 588–589.
Roshdy, M.A. and Coons, L.B., 1975. The subgenusPersicargas (Ixodoidea: Agrasidae: Argas). 23. Fine structure of the salivary glands of unfedA. (P). arboreus Kaiser, Hoogstral, and Kohls. J. Parasitol. 61: 743–752.
Rudolph, D. and Knülle, W., 1974. Site and mechanism of water vapour uptake from the atmosphere in ixodid ticks. Nature, 249: 84–85.
Rudolph, D. and Knülle, W., 1978. Uptake of water vapour from the air:process, site and mechanism in ticks. In: K. Achmidt-Nielsen, L. Bolis and S.H.P. Maddrell (Editors), Comparative Physiology — Water, Ions and Fluid Mechanics. Cambridge University Press, Cambridge, pp. 97–113.
Sigal, M.D., 1990. Ph.D. Dissertation, The Ohio University, Columbus, 181 pp.
Spurr, A.R., 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res., 26: 31–43.
Venable, J.H. and Coggeshall, R., 1965. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol., 24: 407–408.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Needham, G.R., Rosell, R. & Greenwald, L. Ultrastructure of type-I salivary-gland acini in four species of ticks and the influence of hydration states on the type-I acini ofAmblyomma americanum . Exp Appl Acarol 10, 83–104 (1990). https://doi.org/10.1007/BF01194085
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01194085