Abstract
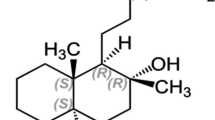
In diethyl ether extracts from celeriac (Apium graveolens L. var.rapaceum) all four stereoisomers of (3a–7a)-cis-3-butylhexahydrophthalide were found to be present. The analyses were carried out by means of GC, enantioselective GC and GC-MS. The assignment of the relative configuration of the diastereomers3 and4 was accomplished by NOE difference spectroscopy.
Similar content being viewed by others
Literatur
Vernin G, Párkányi C (1994) Dev Food Science 34: 329–345
Ciamician G, Silber P (1897) Ber 30: 1419–1424
MacLeod G, Ames JM (1989) Phytochemistry 28: 1817–1824
Van Wassenhove F, Dirinck P, Schamp N (1988). In: Schreier P (Hrsg) Bioflavour 87. de Gruyter, Berlin, S 137–144
Van Wassenhove F, Dirinck P, Vulsteke G, Schamp N (1990) Hort Science 25: 556–559
Van Wassenhove FA, Dirinck PJ, Schamp NM, Vulsteke GA (1990) J Agric Food Chem 38: 220–226
Gijbels MJM, Fischer FC, Scheffer JJC, Baerheim Svendsen A (1985) Fitoterapia 56: 17–23
Bjeldanes LF, Kim I-S (1977) J Org Chem 42: 2333–2335
Lund ED, Wagner CJ Jr, Bryan WL (1973) Proc Florida State Hort Soc 86: 255–259
Wilson CW III (1970) J Food Sci 35: 766–768
Chemwindow3, Version 3.0.0.C13, C-13-NMR Modul von Prof. E. Pretsch. Institut für Organische Chemie der ETH-Zürich, SoftShell International Ltd, 1993
Still WC, Kahn M, Mitra A (1978) J Org Chem 43: 2923–2925
Blum W, Aichholz R (1990) J High Res Chromat 13: 515–518
Canonne P, Akssira M (1985) Tetrahedron 41: 3695–3704
Eggelte TA, de Koning H, Huisman HO (1977) Rec Trav Chim Pays-Bas 96: 271–275
Suzuki H, Tanaka A, Yamashita K (1987) Agric Biol Chem 51: 3369–3373
Tanaka A, Suzuki H, Yamashita K (1989) Agric Biol Chem 53: 2253–2256
Nagai U, Mitsuhashi H (1965) Tetrahedron 21: 1433–1440
Bemelmans JMH (1979) Review of isolation and concentration techniques. In: Land DG, Nursten HE (Hrsg) Progress in flavour research. Applied Science Publishers, London, p 79
Nagai U, Shishido T, Chiba R, Mitsuhashi H (1965) Tetrahedron 21: 1701–1709
Cocker W, McMurry TBH, Sainsbury DM (1966) J Chem Soc (C) 1152–1155
Fujiwara Y, Kimoto S, Okamoto M (1975) Chem Pharm Bull 23: 1396–1403
Pourahmady N, Eisenbraun EJ (1983) J Org Chem 48: 3067–3070
Krischke G (1991) Eignung von Sellerie (Apium graveolens var.rapaceum) als Rohstoff für industrielle Verarbeitung, Diss. TU München
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Manzardo, G.G.G., Kürsteiner-Laube, S. & Perrin, D. Chirale Phthalid-Aromastoffe: (3a–7a)-cis-3-Butylhexahydrophthalid-Stereoisomere in Knollensellerie (Apium graveolens L var.rapaceum). Z Lebensm Unters Forch 203, 501–506 (1996). https://doi.org/10.1007/BF01193153
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01193153