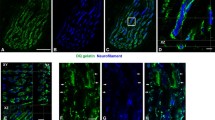
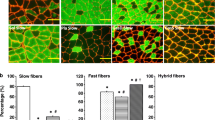
Summary
We have recently observed increase in Type I fibres in mouse soleus — but not extensor digitorum longus — muscles as a result of repeated muscle damage induced by voluntary wheel running. The most likely mechanism underlying the changes in fibre type composition is a redistribution of motor units with axonal sprouting and formation of new synapses. To test this hypothesis we exercised mice on a motor-driven treadmill once (3 × 3 h with 30 min rest periods in between, 14 m min−1, slope 6 °) or repeatedly (8–10 times at intervals of 3–5 days) and quantified axonal sprouting after staining with zinc iodide-osmium. In the contralateral solei, muscle damage and fibre type changes were evaluated with standard histochemical techniques.
Significant numbers of damaged muscle fibres were found 0–15 days after a single exercise as compared to unexercised control animals (range 0.0–0.3% of the fibres in sedentary,n=5,vs 2.1–14.8% in exercised muscles,n=10) and repeated damage occurred in repeatedly exercised animals. In muscles of sedentary animals 3.8 ± 1.4% SD of the examined endplates (n=880, 5 muscles) had nodal or terminal sprouts. The incidence of sprouting was significantly elevated 3–21 days after a single exercise (7.5 ± 1.8%,n=2855, 12 muscles,P < 0.01 signed-rank test), and more so after repeated running (12.0 ± 2.5%,n=1505, 6 muscles,P < 0.01). Fibre type distributions were not different from controls 3 weeks after a single running episode, but after the 6–7 weeks of repeated running a significant increase in undifferentiated fibres at the cost of Type II fibres was found (9.7 ± 3.4% versus 1.0 ± 0.5% in sedentary controls,P < 0.05,t-test); undifferentiated fibres express both Type I and Type II myofibrillar ATPase and are considered as fibres in the process of changing their types. These observations strongly support the assumption that sprouting and formation of new synapses — followed by motor unit enlargement and redistribution — occur as a result of muscle damage.
Similar content being viewed by others
References
Armstrong, R. B. (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review.Medicine and Science in Sports and Exercise 16, 529–38.
Armstrong, R. B., Ogilvie, R. W. &Schwane, J. A. (1983) Eccentric exercise-induced injury to rat skeletal muscle.Journal of Applied Physiology 54, 80–93.
Bartsch, R. C., Mcconnell, E. E., Imes, G. D. &Schmidt, J. M. (1977) A review of exertional rhabdomyolysis in wild and domestic animals and men.Veterinary Pathology 14, 314–24.
Brooks, S. V. &Faulkner, J. A. (1990) Contraction-induced injury: recovery of skeletal muscles in young and old mice.American Journal of Physiology 258 (Cell Physiology 27), C436-C442.
Brown, M. C., Holland, R. L. &Hopkins, W. G. (1981) Motor nerve sprouting.Annual Review of Neuroscience 4, 17–42.
Butler, J. &Cosmos, E. (1981) Enzymic markers to identify muscle-nerve formation during embryogenesis: modified myosin ATPase and silver-cholinersterase histochemical reactions.Experimental Neurology 73, 831–6.
Caroni, P. &Grandes, P. (1990) Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors.Journal of Cell Biology 110, 1307–17.
Covault, J. &Sanes, J. R. (1986) Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle.Journal of Cell Biology 102, 716–30.
Desypris, G. &Parry, D. J. (1990) Relative efficacy of slow and fast alpha-motoneurons to reinnervate mouse soleus muscle.American Journal of Physiology 258 (Cell Physiology 27), C62-C70.
Dickson, G., Peck, D., Moore, S. E., Barton, C. H. &Walsh, F. S. (1990) Enhanced myogenesis in N-CAM-transfected mouse myoblasts.Nature 344, 348–51.
Duchen, L. W. (1970) Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles.Journal of Neurology, Neurosurgery and Psychiatry 33, 40–54.
Geller, S. A. (1973) Extreme exertion rhabdomyolysis. A histopathologic study of 31 cases.Human Pathology 4, 241–50.
Guth, L. &Samaha, F. J. (1970) Procedure for the histochemical demonstration of actomyosin ATPase.Experimental Neurology 28, 365–7.
Hennig, R. &Lømo, T. (1985) Firing patterns of motor units in normal rats.Nature 314, 164–6.
Highman, B. &Altland, P. D. (1963) Effects of exercise and training on serum enzyme and tissue changes in rats.American Journal of Physiology 205, 162–6.
Hikida, R. S., Staron, R. S., Hagermann, F. C., Sherman, W. M. &Costill, D. L. (1983) Muscle fiber necrosis associated with human marathon runners.Journal of Neurological Sciences 59, 185–203.
Holland, R. L. &Brown, M. C. (1981) Nerve growth in botulinum toxin poisoned muscles.Neuroscience 6, 1167–79.
Huang, Ch. L. H. &Keynes, R. J. (1983) Terminal sprouting of mouse motor nerves when the post-synaptic membrane degenerates.Brain Research 274, 225–9.
Irintchev, A. &Wernig, A. (1987) Muscle damage and repair in voluntarily running mice: strain and muscle differences.Cell and Tissue Research 249, 509–21.
Irintchev, A., Draguhn, A. &Wernig, A. (1990) Reinnervation and recovery of mouse soleus muscle after long-term denervation.Neuroscience 39, 231–43.
Jones, D. A., Newham, D. J., Round, J. M. &Tolfree, S. E. J. (1986) Experimental human muscle damage: morphological changes in relation to other indices of damage.Journal of Physiology 375, 435–18.
Kuipers, H., Drukker, J., Frederik, P. M., Geurten, P. &V. Kranenburg, G. (1983) Muscle degeneration after exercise in rats.International Journal of Sports Medicine 4, 45–51.
Lojda, Z., Gossrau, R. &Schiebler, T. H. (1976)Enzymhistochemische Methoden. Berlin, Heidelberg, New York: Springer-Verlag.
Mccully, K. K. (1986) Exercise-induced injury to skeletal muscle.Federation Proceedings 45, 2933–6.
Nachlas, M. M., Tsou, K. C., Desouza, E., Cheng, C. S. &Seligman, A. M. (1957) Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole.Journal of Histochemistry and Cytochemistry 5, 420–36.
Newham, D. J. (1988) The consequences of eccentric contractions and their relationship to delayed onset muscle pain.European Journal of Applied Physiology 57, 353–9.
Pécot-Dechavassine, M. (1986) Increase in polyneuronal innervation in frog muscle after muscle injury.Journal of Physiology 371, 167–77.
Pette, D. &Schnez, U. (1977) Co-existence of fast and slow type myosin light chains in single muscle fibres during transformation as induced by long-term stimulation.FEBS Letters 83, 128–30.
Pette, D. &Staron, R. S. (1990) Cellular and molecular diversities of mammalian skeletal muscle fibres.Reviews of Physiology, Biochemistry and Pharmacology 116, 1–76.
Rich, M. M. &Lichtman, J. W. (1989)In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle.Journal of Neuroscience 9, 1781–805.
Sanes, J. R., Schachner, M. &Covault, J. (1986) Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin, and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle.Journal of Cell Biology 102, 420–31.
Schumann, H. J. (1967) Experimentelle Skelettmuskelnekrosen nach Laufzwang.Gegenbaurs Morphologisches Jahrbuch 111, 107–11.
Staron, R. S. &Pette, D. (1987) Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles.Pflügers Archiv 409, 67–73.
Vihko, V., Salminen, A. &Rantamäki, J. (1978) Acid hydrolase activity in red and white skeletal muscle of mice during a two-week period following exhausting exercise.Pflügers Archiv 378, 99–106.
Vihko, V., Salminen, A. &Rantamäki, J. (1979) Exhaustive exercise, endurance training, and acid hydrolase activity in skeletal muscle.Journal of Applied Physiology 47, 43–50.
Wernig, A. &Dorlöchter, M. (1989) Plasticity of the nerve muscle junction. InFundamentals of Memory Formation: Neuronal Plasticity and Brain Function. Progress in Zoology 37 (edited byRahmann, M.) pp. 83–99. Stuttgart & New York: Gustav Fischer Verlag.
Wernig, A. &Herrera, A. A. (1986) Sprouting and remodelling at the nerve-muscle junction.Progress in Neurobiology 27, 251–91.
Wernig, A. &Irintchev, A. (1989) Sprouting and remodelling at the nerve-muscle junction. InPeripheral Neuropathies 1988. What is significantly new? Fidia Research Series Vol. 21 (edited byAssal, J. -Ph. &Liniger, C.) pp. 57–88. Padova: Liviana Press; Berlin, Heidelberg, New York, Tokyo: Springer Verlag.
Wernig, A., Pécot-Dechavassine, M. &Stover, H. (1980) Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare.Journal of Neurocytology 9, 277–303.
Wernig, A., Carmody, J. J., Anzil, A. P., Hansert, E., Marciniak, M. &Zucker, H. (1984) Persistence of nerve sprouting with features of synapse remodelling in soleus muscles of adult mice.Neuroscience 11, 241–53.
Wernig, A., Irintchev, A. &Weisshaupt, P. (1990) Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running.Journal of Physiology 428, 639–52.
Wernig, A., Salvini, T. F., Langenfeld-Oster, B., Irintchev, A. &Dorlöchter, M. (1992) Endplate and motor unit remodelling in vertebrate muscles. InPlasticity of Motoneuronal Connections (edited byWernig, A.) Amsterdam: Elsevier, in press.
Zerba, E., Komorowski, T. E. &Faulkner, J. A. (1990) Free radical injury to skeletal muscles of young, adult, and old mice.American Journal of Physiology 258 (Cell Physiology 27), C429-C435.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wernig, A., Salvini, T.F. & Irintchev, A. Axonal sprouting and changes in fibre types after running-induced muscle damage. J Neurocytol 20, 903–913 (1991). https://doi.org/10.1007/BF01190468
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01190468