Summary
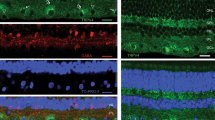
An immunoreaction against glutamate was used to visualize photoreceptors, bipolar, and ganglion cells in the turtle retina. Incubation of the retina prior to fixation in kainic acid (9 μm) led to selective loss of glutamate-like immunoreactivity in OFF-centre bipolar cells, as judged by the loss of staining in the distal half of the inner plexiform layer. In addition, displaced bipolar cells and ganglion cells lost their immunoreactivity. Incubation of the retina in 2,3-cis piperidine dicarboxylate (1mm) did not result in noticeable glutamate depletion in any cell but enhanced labelling in displaced bipolar cells. These findings suggest that all displaced bipolar cells in the turtle retina are depolarized by kainic acid and hyperpolarized by 2,3-cis piperidine dicarboxylate.
Similar content being viewed by others
References
Ali, M. A. &Anctil, M. (1976)Retinas of Fishes. Berlin, Heidelberg, New York: Springer-Verlag.
Ammermüller, J., Muller, J. &Kolb, H. (1995.) The organization of the turtle inner retina. II. Analysis of colour-coded and directionally selective cells.Journal of Comparative Neurology, in press.
Brown, K. T. (1969) A linear area centralis extending across the turtle retina and stabilized to the horizon by non-visual clues.Vision Research 9, 1053–62.
Detwiler, P. B. &Sarthy, P. V. (1981) Selective uptake of lucifer yellow by bipolar cells in the turtle retina.Neuroscience Letters 22, 227–32.
Ehinger, B., Ottersen, O. P., Storm-Mathisen, J. &Dowling, J. E. (1988) Bipolar cells in the turtle retina are strongly immunoreactive for glutamate.Proceedings of the National Academy of Sciences (USA) 85, 8321–5.
Ehrlich, D. &Morgan, I. G. (1980) Kainic acid destroys displaced amacrine cells in the post-hatch chicken retina.Neuroscience Letters 17, 43–8.
Famiglietti, E. V., Kaneko, A. &Tachibana, M. (1977) Neuronal architecture of ON and OFF pathways to ganglion cells in carp retina.Science 198, 1267–9.
Hepler, J. R., Toomin, C. S., McCarthy, K. D., Conti, C. F., Battaglia, G., Rustioni, A. &Petrusz, P. (1988) Characterization of antisera to glutamate and aspartate.Journal of Histochemistry and Cytochemistry 36, 13–22.
Hurd, L. B., II &Eldred, W. D. (1989) Localization of GABA and GAD-like immunoreactivity in the turtle retina.Visual Neuroscience 3, 9–20.
Ingham, C. A. &Morgan, I. G. (1983) Dose-dependent effects of intravitral kainic acid on specific cell types in chicken retina.Neuroscience 9, 165–81.
Kleinschmidt, J., Zucker, C. L. &Yazulla, S. (1986) Neurotoxic action of kainic acid in the isolated toad and goldfish retina: I. Description of effects.Journal of Comparative Neurology 254, 184–95.
Kolb, H. (1982) The morphology of the bipolar cells, amacrine cells and ganglion cells in the retina of the turtlePseudemys scripta elegans.Philosophical Transactions of the Royal Society of London, B 298, 355–93.
Kouyama, N. &Ohtsuka, T. (1985) Quantitative morphological study of the outer nuclear layer in the turtle retina.Brain Research 345, 200–3.
Marc, R. E., Liu, W. -L. S., Kalloniatis, M., Raiguel, S. F. &Van Haesendonck, E. (1990) Patterns of glutamate immunoreactivity in the goldfish retina.Journal of Neuroscience 10, 4006–34.
Matsumoto, N. &Naka, K. -I. (1972) Identification of intracellular responses in the frog retina.Brain Research 42, 59–71.
Miller, R. F. &Slaughter, M. M. (1986) Excitatory amino acid receptors of the retina. Diversity of subtypes and conductance mechanisms.Trends in Neurosciences 9, 211–18.
Morgan, I. G. &El-Lakany, S. (1982) Folic acid derivatives do not reproduce the neurotoxic effects of kainic acid on chicken retina.Neuroscience Letters 34, 69–73.
Morgan, I. G. &Ingham, C. A. (1981) Kainic acid effects both plexiform layers of chicken retina.Neuroscience Letters 21, 275–80.
Pow, D. V. &Robinson, S. R. (1994) Glutamate in some retinal neurons is solely derived from glia.Neuroscience 60, 355–66.
Ramon Y Cajal, S. (1892) La rétine des vertébrés.La Cellule 9, 121–225.
Richter, A. &Simon, E. J. (1975) Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina.Journal of Physiology 248, 317–34.
Schütte, M. (1988) Die funktionelle Morphologie der serotonergen Structuren in der Schildkröten Retina. PhD Thesis, Ludwig-Maximilians-Universität, Munich.
Schütte, M. &Schlemermeyer, E. (1993) Depolarization elicits, while hyperpolarization blocks uptake of endogenous glutamate by retinal horizontal cells of the turtle.Cell and Tissue Research 274, 553–8.
Schütte, M. &Weiler, R. (1987) Morphometric analysis of serotonergic bipolar cells in the retina and its implications for retinal image processing.Journal of Comparative Neurology 260, 619–26.
Schütte, M. &Witkovsky, P. (1991) Dopaminergic interplexiform cells and centrifugal fibres in theXenopus retina.Journal of Neurocytology 20, 195–207.
Sherry, D. M. &Ulshafer, R. J. (1992) Neurotransmitter-specific identification and characterization of neurons in the all-cone retina ofAnolis carolinensis. II. Glutamate and aspartate.Visual Neuroscience 9, 313–23.
Slaughter, M. M. &Miller, R. F. (1983) An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons.Science 219, 1230–2.
Stone, S. &Schütte, M. (1991) Physiological and morphological properties of OFF- and ON-center bipolar cells in theXenopus retina. Effects of glycine and GABA,Visual Neuroscience 7, 363–76.
Tauchi, M. (1990) Single cell shape and population densities of indoleamine-accumulating and displaced bipolar cells in Reeves' turtle retina.Proceedings of the Royal Society of London B238, 351–67.
Weiler, R. (1981) The distribution of center-depolarizing and center-hyperpolarizing bipolar cell ramifications within the inner plexiform layer of the turtle retina.Journal of Comparative Physiology 144, 459–64.
Weiler, R. &Schütte, M. (1985a) Morphological and pharmacological analysis of putative serotonergic bipolar cells in the retina of a turtle,Pseudemys scripta elegans.Cell and Tissue Research 241, 373–82.
Weiler, R. &Schütte, M. (1985b) Kainic acid induced release of serotonin from OFF-center bipolar cells in the turtle retina.Brain Research 360, 379–83.
Yaqub, A. &Eldred, W. D. (1993) Effects of excitatory amino acids on immunocytochemically identified populations of neurons in turtle retina.Journal of Neurocytology 22, 644–662.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schütte, M. Effects of kainic acid and piperidine dicarboxylic acid on displaced bipolar cells in the turtle retina. J Neurocytol 24, 361–369 (1995). https://doi.org/10.1007/BF01189063
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01189063