Summary
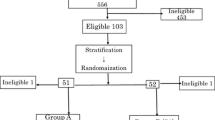
The response and survival of 26 patients with liver metastases from breast cancer, who received OK-432-combined adoptive immunotherapy from 1984 to 1990, were evaluated. OK-432-combined adoptive immunotherapy was comprised sequential treatment via the hepatic artery with a streptococcal preparation, OK-432 (1–5 KE), and adoptive transfer of lymphocytes expanded in T-cell growth factor and sonicated tumor extract antigen. Seventeen (65%) patients responded to the therapy. The median survival time of all patients after treatment was 13 months (range, 2–63 months). Of the 20 prognostic factors analyzed, performance status (PS) alone was related to response (P<0.01). The response rate of the patients with a PS of 0–2 was 83% but only 25% in those with a PS of 3 or 4. In univariate analysis, 11 factors significantly influenced the survival: tumor response; size of primary tumor; menopausal status; PS; serum bilirubin, albumin, lactate dehydrogenase and glutamate-oxalate transaminase (aspartate aminotransferase); the extent of liver involvement; and the number and the proliferation rate of transferred lymphocytes. The MST was 22.8 months for the responders versus 2.8 months for the nonresponders (P<0.01). In multivariate analysis, the most important factor associated with survival was the tumor response, as well as PS, liver involvement, lactate dehydrogenase and albumin. These results suggest that OK-432-combined adoptive immunotherapy can be considered a candidate for a randomised control study and these factors should be used for stratification.
Similar content being viewed by others
Abbreviations
- TCGF:
-
T-cell growth factor
- PS:
-
performance status
- MST:
-
median survival time
- CR:
-
complete response
- PR:
-
partial response
- NC:
-
no change
- PD:
-
progressive disease
References
Clark GM, Sledge GW, Osborne CK, McGuire WL (1987) Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol 5:55–61
Cox DR (1972) Regression models and life tables. J R Statist Soc [B] 34:187–220
Gehan EA (1965) A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52:203–224
George SL, Hogstraten B (1978) Prognostic factors in the initial response to therapy by patients with advanced breast cancer. J Natl Cancer Inst 60:731–736
Guillou PJ (1987) Potential impact of immunobiotechnology on cancer therapy. Br J Surg 74:705–710
Inamoto T, Ohgaki K, Hikasa Y, Kodama H (1984) Specific antitumor immunity in pre- and postoperative state and five year survival of breast cancer patients. Arch Jpn Chir 53:345–352
Kamby C, Bruun RB, Kristensen B (1989) Oestrogen receptor status of primary breast carcinomas and their metastases. Relation to pattern of spread and survival after recurrence. Br J Cancer 60:252–257
Kan N, Ohgaki K, Inamoto T, Kodama H (1984) Anti-tumor and therapeutic effects of spleen cells from tumor-bearing mice cultured with T cell growth factor and soluble tumor extract. Cancer Immunol Immunother 18:215–222
Kan N, Okino T, Kodama H, Ohgaki K, Tobe T, Inamoto T (1987) Breast cancer-specific immunity evaluated by a new in vitro method; IL-2 enhanced MLTR. J Jpn Surg Soc 88:1624–1631
Kan N, Okino T, Nakanishi M, Sato K, Mise K, Yamasaki S, Teramura Y, Ohgaki K, Tobe T (1989) Therapeutic efficacy of sequential therapy with OK-432, cyclophosphamide, IL2-cultured lymphocytes and in vivo IL2 against advanced murine plasmacytoma. Biotherapy 1:197–206
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Statist Assoc 53:457–481
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Nakanishi M, Kan N, Okino T, Satoh K, Mise K, Teramura Y, Yamasaki S, Ohgaki K, Tobe T (1990) Comparison between crude interleukin-2 and recombinant interleukin-2 in maintaining killing activity of cultured lymphocytes. Biotherapy 2:21–32
Okamoto H, Monami M, Shoin S, Koshimura S, Shimizu R (1966) Experimental anticancer studies part 31. On the streptococcal preparation having potent anticancer activity. Jpn J Exp Med 36:175–186
Okino T, Kan N, Nakanishi M, Satoh K, Mise K, Teramura Y, Yamasaki S, Hori T, Kodama H, Ohgaki K, Tobe T (1990) The therapeutic effect of OK-432-combined adoptive immunotherapy against liver metastases from breast cancer. J Cancer Res Clin Oncol 116:197–202
Peetz M, Swanson J, Moseley HS, Fletcher WS (1982) Endocrine ablation and hepatic artery infusion in the treatment of metastases to the liver from carcinoma of the breast. Surg Gynecol Obstet 155:395–400
Powles TJ, Coombes RC, Smith IE, Mary Jones J, Ford HT, Gazet JC (1980) Failure of chemotherapy to prolong survival in a group of patients with metastatic breast cancer. Lancet 1:580–582
Rosenberg SA, Lotze MT, Muul LN, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, Seipp CA, Simpson CG, White DE (1987) A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 316:889–897
Ross MB, Buzdar AU, Smith TL (1985) Improved survival of patients with metastatic breast cancer receiving combination chemotherapy. Cancer 55:341–346
Stehlin JS, De Ipolyi PD, Greeff P, McGaff CJ, Davis BR, McNary L (1988) Treatment of cancer of the liver: twenty years' experience with infusion and resection in 414 patients. Ann Surg 208:23–35
Tormey DC, Kline JC, Palta M (1985) Short term high density systemic therapy for metastatic breast cancer. Breast Cancer Res Treat 5:177–188
WHO Handbook for Standardized Cancer Registries. WHO Offset publication no. 25, World Health Organization, Geneva
Zinser JW, Hortobagyi GN, Buzdar AU, Smith TL, Fraschini G (1987) Clinical course of breast cancer patients with liver metastases. J Clin Oncol 5:773–782
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamasaki, S., Okino, T., Kan, N. et al. Factors influencing the response and survival of patients with liver metastases from breast cancer receiving OK-432-combined adoptive immunotherapy. J Cancer Res Clin Oncol 118, 157–162 (1992). https://doi.org/10.1007/BF01187506
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01187506