Abstract
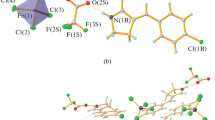
The molecule of the 2∶1 salt consists of two quinidine cations and the 5,5′-dinitrodiphenic anion. Both of the quinidine cations are protonated at the nitrogen atoms of the quinuclidine fragments. Owing to the interlocking hydrogen bonds between quinidine and the dinitrodiphenic ions, a rigid structure of the salt molecule has been formed. The alkaloid molecular conformation around the C(8)-C(9) bond is “open.” The conformational parameters of 5,5′-dinitrodiphenic anion are considerably changed in comparison with the conformation of diphenic acid in the solid state. The absolute configuration of biphenyl 5,5′-dinitro-2,2′-dicarboxylic anion (R) is defined by the negative torsion angles around the line which connects the centers of both phenyl rings, and is the reverse of that observed in the diphenic acid-quinine salt. The present data confirm the observation that the chirality of the salt is controlled by the chirality of theCinchona alkaloid molecule.
Similar content being viewed by others
References
Cahn, R. S., Ingold, C. K., and Prelog, V. (1966)Angew. Chem. Int. Ed. Engl.,5, 385.
Gawroński, J., and Gawrońska, K. (1984)J. Chem. Res. (S), 304.
Gawroński, J., Brzostowska, M., and Koput, J. (1989)Croat. Chem. Acta 62, 97.
Grethe, G., Lee, H. L., Mitt, T., and Uskoković, M. R. (1978)J. Am. Chem. Soc. 100, 589.
Jaskólski, M. (1982)Collected Abstracts of the Fourth Symposium on Organic Crystal Chemistry, Poznań, September 1982, Z. Kałuski, (ed.), pp. 70–71. (A. Mickiewicz Univ., Poznań, Poland).
Johnson, C. K. (1976)ORTEPII. Report ORNL-5138. (Oak Ridge National Laboratory, Tenn.).
Kubicki, M., Borowiak, T., Gawron, M., Giel, M., and Gawroński, J. (1990)J. Cryst. Spectr. Res. 20, 447.
Lehmann, M. S., and Larsen, F. K. (1974)Acta Crystallogr. A 30, 580.
Lightner, D. A., Gawroński, J. K., and Wijekoon, W. H. D. (1987)J. Am. Chem. Soc. 109, 6354.
Motherwell, W. D. S., and Clegg, W. (1978)Pluto 78, program for drawing molecular and crystal structures (Univ. of Cambridge, Cambridge, England).
Nardelli, M. (1983)Comput. Chem. 7, 95.
Sheldrick, G. M. (1976)Shelx 76,program for crystal structure determination (University of Cambridge, Cambridge, England).
Sheldrick, G. M. (1986)Shelx S86, program for crystal structure determination (University of Cambridge, Cambridge, England).
Svendsen, J. S., Markó, I., Jacobsen, E. N., Rao, Ch. P., Bott, S., and Sharpless, K. B. (1989)J. Org. Chem. 54, 2264.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubicki, M., Borowiak, T. & Gawron, M. Structure and molecular chirality of the acetonitrile adduct of the 2:1 salt of quinidine with biphenyl 5,5′-dinitro-2,2′-dicarboxylic acid (5,5′-dinitrodiphenic acid). Journal of Crystallographic and Spectroscopic Research 22, 205–211 (1992). https://doi.org/10.1007/BF01186258
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01186258