Abstract
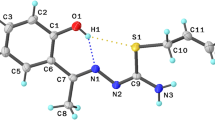
The crystal and molecular structure of thiamine monochloride (C12H17N4OSCl) was determined by X-ray diffraction and refined to a finalR value of 0.042. The compound crystallizes in the monoclinic system, space groupP21/a, with cell constantsa=18.929(4),b=11.663(2),c=6.376(2) Å andβ=96.72(8)°. The thiamine molecule is anhydrous and unprotonated, and the torsion angles at the methylene carbon show that it possesses anF conformation. The dihedral angle value of 84.16(6)° between thiazolium and pyrimidine rings is in the normal range found for the thiamine withF conformation, protonated or not, hydrated or not. Thiamine therefore has the same conformation notwithstanding protonation or hydration, with the rings similarly oriented in all the crystal structures containing thiamine.
Similar content being viewed by others
References
Blank, G., Rodrigues, M., Pletcher, G., and Sax, M. (1976)Acta Crystallogr. B 32, 2970.
Cain, A. H., Sullivan, G. R., and Roberts, J. D. (1977)J. Am. Chem. Soc. 99, 6423.
Clemente, D. A., Bandoli, G., Benetollo, F., and Marzotto, A. (1974)J. Cryst. Mol. Struct. 4, 1.
Cramer, R. E., Maynard, R. B., and Ibers, J. A. (1981)J. Am. Chem. Soc. 103, 76.
Kraut, J., and Reed, H. J. (1962)Acta Crystallogr. 15, 747.
Marzotto, A., (1973)J. Inorg. Nucl. Chem. 35, 3403.
Marzotto, A., (1975)J. Inorg. Nucl. Chem. 37, 1329.
Marzotto, A., and Galzigna, L. (1971)Int. J. Vitam. Res. 41, 401.
Marzotto, A., and Galzigna, L. (1983)Inorg. Chim. Acta 79, 248.
Marzotto, A., Bandoli, G., Clemente, D. A., Benetollo, F., and Galzigna, L. (1973a)J. Inorg. Nucl. Chem. 35, 2769.
Marzotto, A., Manani, G., and Galzigna, L. (1973b)Int. J. Vitam. Res. 43, 3.
Marzotto, A., Biagini Cingi, M., and Clemente, D. A., (1987)Inorg. Chim. Acta 135, 35.
Pletcher, J., and Sax, M. (1972)J. Am. Chem. Soc. 94, 3998.
Pletcher, J., Sax, M., Sengupta, S., Chu, J., and Yoo, C. S., (1972)Acta Crystallogr. B 28, 2928.
Pletcher, J., Sax, M., Blank, G., and Wood, N., (1977)J. Am. Chem. Soc. 99, 1396.
Sheldrik, G. M., (1976)Shelx 76, System of Computer Programs (University of Cambridge, Cambridge).
Suh, I. H., Kim, Y. J., Yoon, Y. K., and Ahn, S. T., (1982)J. Korean Phys. Soc. 15, 114.
Thompson, D. M., and Richardson, M. F., (1977)Acta Crystallogr. B 33, 324.
Watanabe, A., Tasaki, S., Wada, Y., and Nakamaki, H., (1979)Chem. Pharm. Bull. 27, 2751.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clemente, D.A., Marzotto, A. & Valle, G. Crystal and molecular structure of thiamine monochloride. Journal of Crystallographic and Spectroscopic Research 18, 147–156 (1988). https://doi.org/10.1007/BF01181906
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01181906