Summary
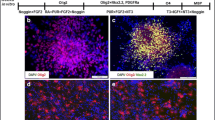
Implantation of hybridoma cells that secrete a monoclonal antigalactocerebroside into the dorsal columns of ⩽ 9-day-old rat spinal cord results in failure of development of dorsal column myelin in the vicinity of the implant. Clusters of apparently undamaged amyelinated axons remain among the hybridoma cells. Ventral myelin is unaffected. These in vivo results support antibody-mediated inhibition of myelin formation as a potential mechanism underlying failure of remyelination in multiple sclerosis.
Similar content being viewed by others
References
Barres, B. A., Hart, I. K., Coles, H. S. R., Burne, J. F., Voyvodic, J. T., Richardson, W. D. &Raff, M. C. (1992) Cell death and control of cell survival in the oligodendrocyte lineage.Cell 70, 31–46.
Bansal, R. &Pfeiffer, S. E. (1989) Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids.Proceedings of the National Academy of Sciences (USA) 86, 6181–5.
Bansal, R., Warrington, A. E., Gard, A. L., Ranscht, B. &Pfeffier, S. E. (1989) Multiple and novel specificities of monoclonal antibodies 01, 04, and R-mAb used in the analysis of oligodendrocyte development.Journal of Neuroscience Research 24, 548–57.
Blakemore, W. F. (1974) Remyelination of the superior cerebellar peduncle in old mice following demyelination induced by cuprizone.Journal of the Neurological Sciences 22, 121–6.
Blakemore, W. F. (1978) Observations on remyelination in the rabbit spinal cord following demyelination induced by lysolecithin.Neuropathology and Applied Neurobiology 4, 47–59.
Blakemore, W. F., Eames, R. A., Smith, K. J. &Mcdonald, W. I. (1977) Remyelination in the spinal cord ofthecat following intraspinal injections of lysolecithin.Journal of the Neurological Sciences 33, 31–43.
Bornstein, M. &Raine, C. S. (1970) Experimental allergic encephalomyelitis. Antiserum inhibition of myelinationin vitro.Laboratory Investigation 23, 536–42.
Bornstein, M. &Raine, C. S. (1977) Multiple sclerosis and experimental allergic encephalomyelitis: specific demyelinationin vitro.Neuropathology and Applied Neuro biology 3, 359–67.
Dorfman, S. H., Fry, J. M., Silverberg, D. H., Grose, C. &Manning, C. (1978) Cerebroside antibody titers in antisera capable of myelination inhibition and demyelination.Brain Research 147, 410–15.
Dubois-Dalcq, M., Niedieck, B. &Buyse, M. (1970) Action of anti-cerebroside sera on myelinated nervous tissue culture.Pathologia Europaea 5, 331–17.
Dyer, C. A. &Benjamins, J. A. (1989) Organization of oligodendroglial membrane sheets: II. Galactocerebroside: antibody interactions signal changes in cytoskeleton and myelin basic protein.Journal of Neuroscience Research 24, 212–21.
Fry, J. M., Weissbarth, S., Lehrer, G. M. &Bornstein, M. B. (1974) Cerebroside antibody inhibits sulfatide synthesis and myelination and demyelinates in cord tissue cultures.Science 183, 540–2.
Hirano, A., Cook, S. D., Whitaker, J. N., Dowling, P. C. &Murray, M. (1971) Fine structural aspects of demyelination in vitro. The effects of Guillain-Barre serum.Journal of Neuropathology and Experimental Neurology 30, 249–65.
Hruby, S., Alvord, Jr, E. C. &Seil, F. J. (1977) Synthetic galactocerebrosides evoke myelination-inhibiting antibodies.Science 195, 173–5.
Kerlero DE Rosbo, N., Honegger, P., Lassmann, H. &Matthieu, J. M. (1990) Demyelination induced in aggregating brain cell cultures by a monoclonal antibody against myelin/oligodendrocyte glycoprotein.Journal of Neurochemistry 55, 583–7.
Pidlesden, S., Lassmann, H., Laffafian, I., Morgan, B. P. &Linington, C. (1991) Antibody-mediated demyelination in experimental allergic encephalomyelitis is independent of complement membrane attack complex formation.Clinical and Experimental Immunology 83, 245–50.
Prineas, J. C. (1985) The neuropathology of multiple sclerosis. InHandbook of Clinical Neurology, Vol 3 (edited byKoester, J. C.) pp. 213–57. Amsterdam: Elsevier.
Prineas, J. C. &Connell, F. (1979) Remyelination in multiple sclerosis.Annals of Neurology 5, 22–31.
Raine, C. S., Hummelgard, A., Swanson, E. &Bornstein, M. B. (1973) Multiple sclerosis: seruminduced demyelinationin vitro: a light and electron microscopic study.Journal of the Neurological Sciences 20, 127–48.
Raine, C. S., Johnson, A. S., Marcus, D., Suzuki, A. &Bornstein, M. B. (1981) Demyelinationin vitro: absorption studies demonstrating that galactocerebroside is a major target.Journal of the Neurological Sciences 52, 117–31.
Ranscht, B., Clapshaw, P. A., Price, J., Noble, M. &Seifert, W. (1982) Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside.Proceedings of the National Academy of Sciences (USA) 79, 2709–13.
Ranscht, B., Wood, P. L. &Bunge, R. P. (1987) Inhibition ofin vitro peripheral myelin formation by monoclonal antigalactocerebroside.Journal of Neuroscience 7, 2936–47.
Rosenbluth, J. (1979) Aberrant axon-Schwann cell junctions in dystrophic mouse nerves.Journal of Neurocytology 8, 655–72.
Rosenbluth, J. (1987) Abnormal axoglial junctions in the myelin-deficient rat mutant.Journal of Neurocytology 16, 497–509.
Rosenbluth, J., Hasegawa, M., Shirasaki, N., Rosen, C. L. &Liu, Z. (1990) Myelin formation after transplantation of normal foetal spinal cord fragments or cultures into myelin-deficient rat spinal cord.Journal of Neurocytology 19, 718–30.
Rosenbluth, J., Liu, Z., Guo, D. &Schiff, R. (1992) Effect of anti-GalC on CNS myelin formation.Society for Neuroscience Abstracts 18, 1449.
Rosenbluth, J., Sumner, A. &Saida, T. (1981) Dedifferentiation of the axolemma associated with demyelination. InProceedings, 39th EMSA Meeting (edited byBailey, G. W.) pp. 496–7. Baton Rouge: Claitor's.
Saida, K., Saida, T., Brown, M. J. &Silverberg, D. H. (1979)In vivo demyelination induced by intraneural injection of antigalactocerebroside serum.American Journal of Pathology 95, 99–110.
Sommer, I. &Schachner, M. (1981) Monoclonal antibodies (01 to 04) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system.Developmental Biology 83, 311–27.
Warrington, A. E., Barbarese, E. &Pfeiffer, S. (1993) Differential myelinogenetic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts.Journal of Neuroscience Research 34, 1–13.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenbluth, J., Liu, Z., Guo, D. et al. Inhibition of CNS myelin developmentin vivo by implantation of anti-GalC hybridoma cells. J Neurocytol 23, 699–707 (1994). https://doi.org/10.1007/BF01181644
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01181644