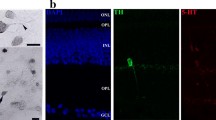
Summary
Excitatory amino acids play an important role in visual processing in the retinas of many species, but little is known about the identity of the specific postsynaptic cell types and the pharmacology of their receptors. To investigate which specific cell types were affected by excitatory amino acids, we examined the effects of exogenous aspartate, glutamate, kainic acid,n-methyl-D-aspartate, and MK-801 on retinal neurons. Specific populations of neurons were labelled using antibodies directed against glucagon, enkephalin, neurotensin, γ-aminobutyric acid, glutamic acid decarboxylase, serotonin, glycine, glutamate or aspartate. We analyzed a combination of long-termin vivo injections (seven days following an intraocular injection of kainic acid) and short term in vitro incubations. There were changes in the labelling intensity and sometimes in the relative localization of all of the antigens in the drug treated retinas. Some observations suggested that the drugs were altering neurotransmitter metabolism. Differential responses were seen in specific cell types within the populations of neurons with neurotensin-, glutamate-, aspartate-, glycine, γ-aminobutyric acid-, and glutamic acid decarboxylase-like immunoreactivity. The immunocytochemical approach used in these studies was able to determine specific retinal cell types which were influenced by particular exitatory amino acids. The broad extent of cell types influenced and the potential metabolic effects suggest that excitatory amino acids and their receptors play a complex role in visual processing.
Similar content being viewed by others
References
Anderton, P. J. &Millar, T. J. (1989) MK-801-induced antagonism of NMDA-preferring excitatory amino acid receptors in horizontal cells of the turtle retina.Neuroscience Letters 101, 331–6.
Cooper, E. J., Millar, T. J. &Anderton, P. J. (1990) Distribution of [3H]MK-801 binding in the turtle retina: and autoradiographical study.Neuroscience Letters 112, 127–32.
Coyle, J. T., Biziere, K. &Schwarcz, R. (1978) Neurotoxicity of excitatory amino acids in the neural retina. InKainic Acid as a Tool in Neurobiology (edited byMcGeer, E. G., McGeer, P. &Olney, J. W.), pp. 177–88. New York, Raven Press.
Coyle, J. T. (1983) Neurotoxic action of kainic acid.Journal of Neurochemistry 41, 1–11.
De Nardis R., Sattayasai J., Zappia J. &Ehrlich D. (1988) Neurotoxic effects of kainic acid on developing chick retina.Developmental Neuroscience 10, 256–69.
Dvorak, D. R. &Morgan, I. J. (1983) Intravitreal kainic acid permanently eliminates off-pathways from chicken retina.Neuroscience Letters 36, 249–53.
Ehinger, B., Ottersen, O. P., Storm-Mathisen, J. &Dowling, J. E. (1988) Bipolar cells in the turtle retina are strongly immunoreactive for glutamate.Proceedings of the National Academy of Sciences (USA) 85, 8321–5.
Ehrlich, D. &Morgan, I. G. (1980) Kainic acid destroys displaced amacrine cells in the post-hatch chicken retina.Neuroscience Letters 17, 43–8.
Ehrlich, D., Teuchert, G. &Morgan, I. G. (1987) Specific ganglion cell death induced by intravitreal kainic acid in the chicken retina.Brain Research 415, 342–6.
Eldred, W. D. &Karten, H. J. (1983) Characterization and quantification of peptidergic amacrine cells in the turtle retina: enkephalin, neurotensin, and glucagon.Journal of Comparative Neurology 221, 371–81.
Eldred, W. D. &Carraway, R. E. (1987) Neurocircuitry of two types of neurotensin containing amacrine cells in the turtle retina.Neuroscience 2, 331–8.
Ferkany, J. W., Zaczek, R. &Coyle, J. T. (1982) Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors.Nature 298, 757–9.
Floyd, D. N., Ashall, F. &Djamgoz, M. B. A. (1992) Protease activities in carp retina.Neurochemistry International 21, 527–33.
Garthwaite, J. &Wilkin, G. P. (1982) Kainic acid receptors and neurotoxicity in adult and immature rat cerebellar slices.Neuroscience 7, 2499–514.
Gibson, B. L. &Reif-Lehrer, L. (1984)In vitro effects of kainate on embryonic and posthatching chick retina.Developmental Brain Research 15, 97–103.
Hampton, C. K., Garcia, C. &Redburn, D. A. (1981) Localization of kainic acid-sensitive cells in mammalian retina.Journal of Neuroscience Research 6, 99–111.
Hurd, L. B. &Eldred, W. D. (1989) Localization of GABA-and GAD-like irnmunoreactivity in the turtle retina.Visual Neuroscience 3, 9–20.
Hurd, L. B., II &Eldred, W. D. (1993) Synaptic microcircuitry of bipolar and amacrine cells with serotonin-like immunoreactivity in the retina of the turtle,Pseudemys scripta elegans.Visual Neuroscience 10, 455–72.
Ingham, C. A. &Morgan, I. G. (1983) Dose-dependent effects of intravitreal kainic acid on specific cell types in chicken retina.Neuroscience 9, 165–81.
Kemp, J. A., Foster, A. C. &Wong, E. H. F. (1987) Non-competitive antagonists of excitatory amino acid receptors.Trends in Neuroscience 10, 294–8.
Kleinschmidt, J., Zucker, C. L. &Yazulla, S. (1986a) Neurotoxic action of kainic acid in the isolated toad and goldfish retina: I. Description of effects.Journal of Comparative Neurology 254, 184–95.
Kleinschmidt, J., Zucker, C. L. &Yazulla, S. (1986b) Neurotoxic action of kainic acid in the isolated toad and goldfish retina: II. Mechanism of action.Journal of Comparative Neurology 254, 196–208.
Kolb, H., Cline, C., Wanh, H. H. &Brecha, N. (1987) Distribution and morphology of dopaminergic amacrine cells in the retina of the turtle (Pseudemys scripta elegans).Journal of Neurocytokgy 16, 577–88.
London, E. D. &Coyle, J. T. (1979) Specific binding of [3H]-kainic acid to receptor sites in rat brain.Molecular Pharmacology 15, 492–505.
Lopez-Colome, A. M. (1981) High-affinity binding ofl-glutamate to chick retinal membranes.Neurochemistry Research 6, 1019–33.
Lopez-Colome, A. M. &Somohano, F. (1984) Localization ofl-glutamate andl-aspartate synaptic receptors in chick retinal neurons.Brain Research 298, 159–62.
Lopez-Colome, A. M. &Somohano, F. (1986) Effect of selective lesions on the release of glutamate and aspartate from chick retina.Journal of Neuroscience Research 15, 205–16.
Lopez-Colome, A. M. &Roberts, P. J. (1987) Effect of excitatory amino acid analogue on release of D-3H-Aspartate from chick retina.European Journal of Pharmacology 142, 409–17.
Loscher, W., Annies, R. &Honack, D. (1991) Then-methyl-D-aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats.Neuroscience Letters 128, 191–4.
Mangel, S. C., Ariel, M. &Dowling, J. E. (1982) Effects of amino acids and drugs on response properties of fish H-cells.Society for Neuroscience Abstract 8, 132.
Miller, R. F. &Slaughter, M. M. (1986) Excitatory amino acid receptors of the retina: diversity of subtypes and conductance mechanisms.Trends in Neuroscience 9, 211–18.
Morgan, I. G. &Ingham, C. A. (1981) Kainic acid affects both plexiform layers of chicken retina.Neuroscience Letters 21, 275–80.
Morris, J. F. &Pow, D. V. (1991) Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytosis.The Anatomical Record 231, 437–45.
Normann, R. A., Perlman, I. &Daly, S. J. (1986) The effects of continuous superfusion ofl-aspartate andl-glutamate on horizontal cells of the turtle retina.Vision Research 26, 259–68.
Oertel, W. H., Tappaz, M. L., Kopin, I. J., Ranson, D. H. &Schmechel, D. E. (1980) Production of an antiserum to a rat brain glutamate (GAD)/cysteine sulfinate (CSD) decarboxylase.Brain Research Bulletin 5, 713–19.
Perez, M. T. R. &Davanger, S. (1992) Kainate-induced toxicity in the rabbit retina: effects on GABA and glycineimmunoreactivity.Investigative Ophthalmology and Visual Science (Suppl.),33, 1031.
Tapia, R. &Arias, C. (1982) Selective stimulation of neurotransmitter release from chick retina by kainic and glutamic acids.Journal of Neurochemistry 39, 1169–78.
Verhage, M., McMahon, H. T., Ghijsen, W. E. M., Boomsma, F. &Nicholls, D. G. (1991) Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals.Neuron 6, 517–24.
Weiler, R. &Ball, A. K. (1984) Co-localization of neurotensin-like irnmunoreactivity and3H-glycine uptake system in sustained amacrine cells of turtle retina.Nature 311, 759–61.
Weiler, R. &Schütte, M. (1985a) Morphological and pharmacological analysis of putative serotonergic bipolar and amacrine cells in the retina of a turtle,Pseudemys scripta elegans.Cell and Tissue Research 241, 373–82.
Weiler, R. &SchÜtte, M. (1985b) Kainic acid-induced release of serotonin from OFF-bipolar cells in the turtle retina.Brain Research 360, 379–83.
Witkovsky, P., Eldred, W. D. &Karten, H. J. (1984) Catecholeamine-indoleamine containing neurons in the turtle retina.Journal of Comparative Neurology 228, 217–25.
Yaqub, A. &Eldred, W. D. (1992) Localization of aspartate-like irnmunoreactivity in the retina of the turtle (Pseudemys scripta).Journal of Comparative Neurology 312, 584–98.
Zeevalk, G. D., Hyndman, A. G. &Nicklas, W. J. (1989) Excitatory amino acid-induced toxicity in chick retina: amino acid release, histology, and effects of chloride channel blockers.Journal of Neurochemistry 53, 1610–19.
Zeevalk, G. D. &Nicklas, W. J. (1990) Action of the anti-ischemic agent ifenprodil onn-methyl-D-aspartate and kainate-mediated excitotoxicity.Brain Research 522, 135–9.
Zhang, D. &Eldred, W. D. (1992) Colocalization of enkephalin-, glucagon-, and corticotropin releasing factor-like immunoreactivity in GABAergic amacrine cells in turtle retina.Brain Research 596, 46–57.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yaqub, A., Eldred, W.D. Effects of excitatory amino acids on immunocytochemically identified populations of neurons in turtle retina. J Neurocytol 22, 644–662 (1993). https://doi.org/10.1007/BF01181490
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01181490