Conclusions
-
1.
Using differential thermal and x-ray structural analyses, the binary systems KCl−UO2Cl2 and NaCl−UO2Cl2 have been studied and their melting diagrams constructed.
-
2.
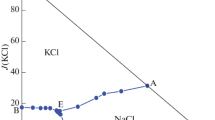
The liquidus surface has been constructed for the NaCl−KCl−UO2Cl2 ternary system and the region of existence of a ternary compound of composition KNa2(UO2)2Cl7 has been found.
Similar content being viewed by others
Literature Cited
R. Robins, J. Nucl. Materials,3, 294 (1961).
R. Robins R. Wilks, and B. Bradburg, J. Nucl. Materials,5, 262 (1962).
F. Scott and L. Mudge, J. Nucl. Materials,9, 245 (1963).
K. Harmon, Metals,13, 670 (1961).
G. Benedict et al., New Nuclear Materials Including Nonmetallic Fuels (Proc. Conf. Prague, 1963), Vol. 1, IAEA, Vienna (1963), p. 21.
M. Schlechter, K. Nucl. Materials,10, 145 (1963).
D. Neumann, Kernenergie,6, 410 (1963).
G. Chauwin and H. Coriou, Cited in NSA-18, 23458 (1964).
HW-SA-3622, 7–9, (1964).
HW-SA-74761 (1962).
HW-SA-2915 (1963).
Homabe Naohiko and Ito Chuko, J. Nucl. Sci.and Technol.,2, 7 (1965).
Ya. M. Sterlin and V. V. Artamonov, Atomnaya Énergiya,22, 473 (1967).
T. Kuroda and T. Suzuki, J. Electrochem. Soc. Japan26, No. 7–9, 140 (1958).
A. Bogaez and W. Tizebiatowski, Roczn. Chem.38, 729 (1964).
V. N. Desyatnik et al., Izv. Vuzov Tsvetn. Metallurg. No. 5, 102 (1966).
N. S. Martynova, I. V. Vasil'kova, and M. P. Susarev, Atomnaya Énergiya,18, 616 (1965).
L. A. Khripin et al., Atomnaya Énergiya,19, 437 (1965).
N. N. Lipilina, Uranium and Its Compounds [in Russian], Moscow, Izd-vo Akad. Nauk SSSR (1959). pp. 176–183.
J. Kats and E. Rabinovich, Chemistry of Uranium [Russian translation], Vol. 1, Izd-vo Inostr. Lit., Moscow (1954), pp. 463–471.
L. Ochs and F. Strassman, L. Naturforsch.,7B, 637 (1952).
E. Yander Wall, D. Bauer, and H. Hahn, IDO-14578 (1963).
M. Lambert, USA Patent, Cl. 23-14.5, No. 3160470 (1964).
Ya. I. Giller, Tables of Interplane Distances [in Russian], Vol. II, Nedra, Moscow (1966).
T. V. Rode, Transactions of the First Conference on Thermography (Kazan', 1953) [in Russian], Izd-vo Akad. Nauk SSSR, Moscow-Leningrad (1955), pp. 154–170.
Diffraction Data Cards and Alphabetical and Grouped Numerical Index of X-Ray Diffraction Data, ASTM, Philadelphia (1946–1963).
Additional information
Translated from Atomnaya Énergiya, Vol. 27, No. 2, pp. 121–124, August, 1969.
Rights and permissions
About this article
Cite this article
Vorobei, M.P., Skiba, O.V., Kapshukov, I.I. et al. Study of salt systems based on NaCl, KCl, and UO2Cl2 . At Energy 27, 827–831 (1969). https://doi.org/10.1007/BF01180283
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01180283