Abstract
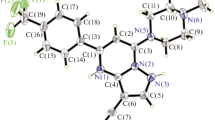
Naphazoline·HNO3 (1): C14H15N3O3,M r =273.3, crystallizes in the orthorhombic space group Pbca(Z=8) witha=12.028(1),b=14.408(2) andc=15.894(2) Å,V=2754.4 Å3,D x =1.318 g cm−3,μ=0.70 mm−1,λ (CuKα)=1.54178 Å,F(000)=1152. FinalR=0.092 andwR=0.088 for 1348 observed reflections collected on a diffractometer. The structure was solved with direct methods, it shows two distinct positions of the naphthyl group with populations about 0.5, which differ by a 180β rotation. As in other arylmethyl-2-imidazolines, the imidazoline ring is approximately perpendicular to the aromatic ring. The molecules are linked by two hydrogen bonds N(3)-H⋯O (nitrate anion)⋯H-N(1) into infinite chains running along the 21 axis in the [100] direction. Tymazoline·HC1·H2O (2): C14H23ClN2O2,M r =286.8, crystallizes in the monoclinic space groupP21/n (Z=4) witha=8.136(1),b=10.015(1),c=18.601(1) Å and,β=97.20(1)°,V=1503.7 Å3 D x =1.266 g cm−3,μ=2.27 mm−1, λ(CuKα)=1.54178 Å,F(000)=636. FinalR=0.115 andwR=0.119 for 3073 observed reflections collected on a diffractometer. The structure was solved with direct methods. Unlike other aryloxymethyl-2-imidazolines, the molecule is almost planar with an angle of 12.8(2)° between the two rings. Hydrogen bonding networks consist of N(1)-H⋯O (water)⋯Cl−⋯H-N(3) chains joined by an additional link O(water)-H⋯Cl− between chains running in opposite directions.
Similar content being viewed by others
References
Cattier-Humblet, C., and Carpy, A. (1985)Eur. J. Med. Chem. 20, 251–255.
Carpy, A., Hickel, D., and Leger, J. M. (1980a)Cryst. Struc. Commun. 9, 37–41.
Carpy, A., Hickel, D., and Leger, J. M. (1980b)Cryst. Struc. Commun. 9, 43–47.
Carpy, A., Leger, J. M., Leclerc, G., Decker, N., Rouot, B., and Wermuth, C. G. (1981)Mol. Pharm. 21, 400–408.
Ghose, S., and Dattagupta, J. K. (1986)Acta Crystallogr. C 42, 1524–1526.
Główka, M. L., and Król, I. (1990)J. Cryst. Spectr. Res. 20, 511–513.
Johnson, C. K. (1976)Ortep.Report ORNL-5138. (Oak Ridge National Laboratory, Tenn.).
Larson, A. C. (1967)Acta Crystallogr. 23, 664–665.
Podder, A., Mukhopadhyay, B. P., Dattagupta, J. K., and Saha, N. N. (1983)Acta Crystallogr. C 39, 495–497.
Sheldrick, G. M. (1976)Shelx.A Program for Crystal Structure Determination. (University Chemical Laboratory, Lensfield Road, Cambridge, England).
Sheldrick, G. M. (1986)Shelxs.A Program for the Solution of Crystal Structures. (University of Göttingen, Federal Republic of Germany).
Walker, N., and Stuart, D. (1983)Acta Crystallogr. A 39, 158–166.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Główka, M.L. Crystal structures of two therapeutically active 2-imidazolines: naphazoline nitrate and tymazoline hydrochloride monohydrate. Journal of Crystallographic and Spectroscopic Research 21, 715–719 (1991). https://doi.org/10.1007/BF01179918
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01179918