Abstract
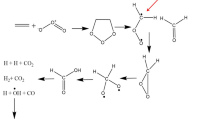
The thermodynamic characteristics of the pyridine N-oxide radical cation were obtained and the energetics of its hydration were estimated. The electrochemically generated pyridine N-oxide radical cations are capable of cleaving an H atom from methanol, which is a component of the medium.
Similar content being viewed by others
Literature cited
Yu. V. Geletii, G. V. Lyubimova, and A. E. Shilov, Kinet. Ratal.,26, No. 4, 1019 (1985).
S. G. Lias, J. F. Liebman, and R. D. Levin, J. Phys. Chem. Ref. Data,13, No. 3, 695 (1984).
R. C. Reid, J. M. Prausnitz, and T. K. Sherwood, The Properties of Gases and Liquids, 3rd ed., McGraw-Hill, New York (1977).
K. P. Mishchenko and A. A. Ravdel' (eds.), A Short Handbook of Physicochemical Values [in Russian], Khimiya, Moscow-Leningrad (1965).
Catalog Handbook of Fine Chemicals, Aldrich Chemical Company, Milwaukee (1984–1985).
H. Miyazaki, T. Rubota, and M. Yamakawa, Bull. Chem. Soc. Jpn.,45, 780 (1972).
S. I. Rulakovskaya, Yu. V. Geletii, L. A. Rushch, et al., Rinet. Katal.,28, No. 4, 1018 (1987).
G. A. Rrestov, Thermodynamics of Ionic Processes in Solutions [Russian translation], Khimiya, Leningrad (1973).
“Tables of rate and equilibrium constants of heterolytic organic reactions,” Itogi Nauki Tekh., 11, Part 1 (1976).
É. D. German and A. M. Ruznetsov., “Theory of the kinetics of processes of electron transfer between complex ions,” Itogi Nauki Tekh., Rinet. Katal.,10, 115 (1982).
A. J. Meyer, J. Mol. Struct. (Theochem.),124, 93 (1985).
V. N. Kondrat'ev (ed.), Energy of Rupture of Chemical Bonds. Ionization Potentials and Electron Affinity [Russian translation], Nauka, Moscow (1974).
Z. Galus, Fundamentals of Electrochemical Analysis, PWN, Warsaw, Poland (1971).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 11, pp. 2438–2441, November, 1989.
We would like to express our deep appreciation to A. E. Shilov for his cooperation in the research and discussion of the results of the study.
Rights and permissions
About this article
Cite this article
Echmaeva, T.A., Kulakovskaya, S.I., Shamaev, S.N. et al. Thermodynamic characteristics of the radical cation of pyridine N-oxide and its reaction with methanol. Russ Chem Bull 38, 2237–2240 (1989). https://doi.org/10.1007/BF01168059
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01168059