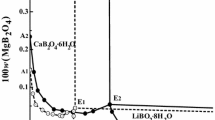
Summary
-
1.
A study of the interaction of aqueous solution of calcium hydroxide with hydrogen peroxide over a wide range of concentrations, carried out by the solubility method applied to the ternary system Ca(OH)2-H2O2-H2O at 0°,−10°, and −21°, has made it possible to determine the actual composition and limits of existence of the stable solid phases.
-
2.
The calcium peroxy compounds whose existence is fully established by the study of the ternary system Ca(OH2-H2O2-H2O at 0°, −10°, and −21° are: CaO2·8H2O; CaO2·2H2O and CaO2·2H2O2.
-
3.
Formation of calcium peroxide octahydrate occurs under restricted conditions at low hydrogen peroxide concentrations.
-
4.
Calcium peroxide diperhydrate is formed over a wide range of hydrogen peroxide concentrations and is relatively stable only at low temperatures.
Similar content being viewed by others
Literature cited
L. G. Thenard, Ann. chim. phys., 8, 306 (1818).
E. Schöne, Experimental Studies of Hydrogen Peroxide, Moscow, 1875.
J. Conroy, J. Chem. Soc., (2) 11, 808 (1873).
R. H. de Forcrand, Comptes rend., 130, 1250 (1900).
R. H. de Forcrand, Comptes rend., 130, 1308 (1900).
R. H. de Forcrand, Comptes rend., 130, 1388 (1900).
E. Riesenfeld, W. Nottebohm, Z. anorg. and allg. Chem., 89, 405 (1914).
W. Traube, and W. Schulze, Ber., 54, 1626 (1921).
V. Kotov and S. Raikhshtein,. Phys. Chem., 15, 9, 1057 (1941).
I. A. Kazarnovsky, J. Phys. Chem., 14, 3, 320 (1940).
P. Ehrlich, Z. anorg. Chem., 252, 370 (1944).
K. V. Astakhov and A. G. Getsov, Proc. Acad. Sci. USSR, 81, No. 1 (1951).
S. Z. Makarov and V. N. Chamova, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1951, No. 3, 255.
S. Z. Makarov and B. A. Lebedev, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1953, No. 1, 58.
Sidell, Solubility of Inorganic and Organic Compounds, Vol II, Van Nostrand Co., 1940, p. 309.
R. T. Haslam, J. Am. Chem. Soc.,, 46, 308 (1924).
H. Bosset, J. Chem. Soc., 1270 (1934).
W. Foley and P. Giguère, Can. J. of Chem., 29, 123 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Makarov, S.Z., Grigoryeva, N.K. Systems containing concentrated hydrogen peroxide Communication 4. Solubility isotherms of the ternary system Ca(OH)2-H2O2-H2O. Russ Chem Bull 3, 323–328 (1954). https://doi.org/10.1007/BF01167806
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01167806