Abstract
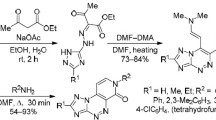
Ring closure of γ-[benzothiazolyl-(2)]-acetoacetates affords benzopyridothiazoles. In the reaction of these substituted acetoacetic acid esters with alkyl halides in the presence of bases, the formation of benzopyridothiazoles is observed; these heterocyclic compounds are alkyl-substituted at C-4. IR- and1H-NMR-spectral data of the benzopyridothiazoles and some of their derivatives are given.
Zusammenfassung
γ-[Benzothiazolyl-(2)]-acetessigsäurealkylester können zu Benzopyridothiazolen cyclisiert werden. Bei Umsetzung solcher substituierter Acetessigester mit Alkylhalogeniden in Gegenwart von Basen erfolgt Cyclisierung zu Benzopyridothiazolen, die an C-4 alkylsubstituiert sind. IR- und1H-NMR-Daten dieser Heterocyclen und einiger ihrer Derivate werden mitgeteilt.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Aus der DissertationN. Hawlitzky, Freiburg im Breisgau, 1970.
Rights and permissions
About this article
Cite this article
Hawlitzky, N., Haller, R. Synthese von 3-Hydroxy-1-oxo-4-alkyl-1H-benzo[d]pyrido[2,1—b]thiazolen. Monatshefte für Chemie 102, 718–723 (1971). https://doi.org/10.1007/BF01167249
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01167249