Abstract
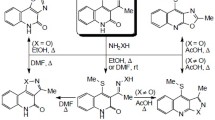
Halogenation, acylation, azo coupling, and alkylaminomethylation of 5,6-dihydroindoto[2,I-alisoguinoline were carried out at the C(11) position (pyrrole fragment). In dibromination, the second bromine atom was introduced into the C(9) position (indole part of the molecule).
Similar content being viewed by others
References
G. Ntaganda, S. A. Soldatova, J. A. R. Alarcon, B. N. Anisimov, and A. T. Soldatenkov, Khim. Geterotsikl. Soedin., No. 1, 83 (1994).
J. A. R. Alarcon, S. A. Soldatova, A. T. Soldatenkov, M. A. Ryashentseva, and N. S. Prostakov, Izv. Akad. Nauk SSSR, Ser. Khim., No. 6, 1413 (1991).
J. E. Saxton, J. Chem. Soc., 3592 (1952).
G. Hart, D. E. Liljegren, and K. T. Potts, J. Chem. Soc., 4267 (1961).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 217–220, February, 1994. Original article submitted November 19, 1993.
Rights and permissions
About this article
Cite this article
Ntaganda, G., Soldatova, S.A., Anisimov, B.N. et al. Indolopyridines with a hetero atom at a position of fusion. 7. Electrophilic substitution in 5,6-dihydroindolo-[2,1-a]isoquinoline. Chem Heterocycl Compd 30, 196–199 (1994). https://doi.org/10.1007/BF01165011
Issue Date:
DOI: https://doi.org/10.1007/BF01165011