Summary
-
1.
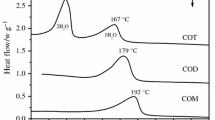
Heating curves with differential registration, using a registering pyrometer, are constructed for the perhydrates Na2CO2·H2O·1,SH2O2 and Na2CO2·H2O·2H2O2. The curves have two inflections, the first being due to an endothermic effect, associated with dehydration, and the second to an exothermic effect, due to thermal decomposition of perhydrate, with evolution of oxygen.
-
2.
The anhydrous perhydrates Na2CO2·1.5H2O2 and Na2CO3·2H2O2 may readily be prepared from the hydrated salts obtained from sodium carbonate and aqueous perhydrol, by heating at temperature exothermic effects, in vacuum.
Similar content being viewed by others
Literature cited
S. Z. Makarov and V. N. Chamova, Bull. Acad. Sci. USSR, Div. Chem. Sci., No. 3, 255 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Makarov, S.Z., Chamova, V.N. Study of systems containing hydrogen peroxide Part 2. Thermal properties and dehydration of perhydrates of sodium carbonate. Russ Chem Bull 1, 589–590 (1952). https://doi.org/10.1007/BF01164922
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01164922