Abstract
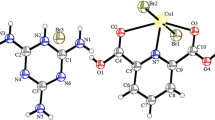
The structure of the Cu(II) complex of gly-l-tyr [Cu(C11N2O4H13) (H2O)2]·2H2O has been solved by X-ray diffraction methods. The compound crystallizes in the orthorhombic space groupP212121 witha=11.970(2) Å,b=12.485(2) Å andc=10.418(3) Å, respectively, λ(MoKα)=0.710 Å,D c =1.59 Mgm−3,D m =1.59 Mgm−3, finalR=0.04. The structure was solved by heavy atom (Cu) phased Fourier and refined by full-matrix least squares methods. The coordination geometry of the ligand around the Cu(II) ion has been established as a distorted tetragonal pyramid. The peptide molecule behaves as a tridentate ligand via its amino (N2), amido (N1) nitrogens and carboxyl (O2) oxygens. The peptide nitrogen is found to be deprotonated.
Similar content being viewed by others
References
Blumberg, W. E.,et al. (eds.), 1966.The Biochemistry of Copper (Proceedings of the symposium on Copper in Bio. Sys. held at Arden House, 1965) (Academic Press, New York).
Busing, W. R., Martin, K. O., and Levy, H. A. (1962)ORFLS, a FORTRAN ctystallographic least-square program, USAEC Report ORNL-TM-305. (Oak Ridge National Laboratory, Oak Ridge, Tenn.).
Franks, W. A., and Van der Helm, D. (1910)Acta Cryst. B 27, 1299.
Freeman, H. C. (1967)Adv. Protein Chem. 22, 257.
International Tables for X-ray Crystallography (1962) Vol. III (Kynoch Press, Birmingham).
Koltan, W. L., Roth, R. H., and Gurd, F. R. N. (1963)J. Biol. Chem. 238, 124.
Mosset, A., and Bonnet, J. J. (1977)Acta Crystallogr. B 33, 2809–2812.
Murali, R., and Subhramanian, E. (1987)Int. J. Peptide Protein Res. 29, 187.
Smith, D. W., and Wiebenga, E. H. (1953)Acta Crystallogr. 6, 531–539.
Solomon, E. I., Hare, J. W., Doo Ley, D. M., Dawson, J. H., Stephens, P. L., and Gray, H. B. (1980)J. Am. Chem. Soc. 102, 171–173. Structure and Bonding, Cu, Mo and V in biological system, Vol. 35 (1983), Springer-Verlag.
Stewart, R. F., Davidson, E. R., and Simpson, W. T. (1965)J. Chem. Phys. 42, 3175.
Tsangaris, J. M., and Martin, R. B. (1970)J. Am. Chem. Soc. 92, 14.
Tsangaris, J. M., Chang, J. W., and Martin, R. B., ibid. (1969)91, 726.
Neurath, H. (1970)The Proteins, Vol. V (second edition) (Academic Press, New York).
Zalkin, A. (1965)FORDAP, a FORTRAN program for Fourier calculations. (Univ. of California, Barkley).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dey, I., Mukhopadhyay, B.P., Das, B.N. et al. Structure of a Cu(II)-containing peptide, diaquo-gly-l-tyr Cu(II) dihydrate, [Cu(C11N2O4H13)(H2O)2·2H2O. Journal of Crystallographic and Spectroscopic Research 23, 65–68 (1993). https://doi.org/10.1007/BF01161290
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01161290