Abstract
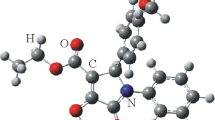
The crystal and molecular structures of two configurational isomers (hereinafter HM and LM) of the title compound have been determined from single-crystal X-ray diffraction data. The HM isomer has crystallographically imposed ¯1 symmetry; then, the two asymmetric carbon atoms must have opposite chirality and the product is the 2R2′S-meso (or 2S2/t'r-meso) stereoisomer. The ethylenediamine group assumes the anti-periplanar conformation, the two phenyl rings are strictly planar, and the two thiazolidine rings present the same envelope conformation. The LM isomer is characterized by a-syn-clinal conformation of the ethylenediamine group, by the same envelope conformation of the two thiazolidine rings, and by the same chirality of the two asymmetric carbons atoms. Nevertheless in the crystal structure both enantiomers, 2R2′R and 2S2′S, are present.
Similar content being viewed by others
References
Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G., and Taylor, R. (1987)J.C.S. Perkin Trans. 510.
Benetollo, F., Bombieri, G., Del Pra, A., Previtera, T., Vigorita, M. G., and Basile, M. (1990)Acta Crystallogr. C 46, 1471.
Bruno, G., Bombieri, G., Del Pra, A., Previtera, T., Vigorita, M. G., and Basile, M. (1984)Acta Cyrstallogr. C 40, 671.
Cremer, D., and Pople, J. A. (1975)J. Am. Chem. Soc. 97, 1354.
Duax, W. L., Weeks, C. M., and Rohrer, D. C. (1976)Stereochemistry, (Wiley, New York), Vol. 9, pp. 284–286.
Hickel, D., Leger, J. M., Carpy, A., Vigorita, M. G., Chimirri, A., and Grasso, S. (1983)Acta Crystallogr. C 39, 240.
Hull, S. E., Viterbo, D., Woolfson, M. M., and Shao-Hui, Z. (1981)Magex.A system of computer programs for the automatic solution of crystal structure from X-ray diffraction data. (Univ. of York, England).
Johnson, C. K. (1976)Ortep. Report ORNL-5138 (Oak Ridge National Laboratory, Tenn.).
Kemmish, H. J., and Hamor, A. T. (1990)Acta Crystallogr. C 46, 246.
Klyne, W., and Prelog, V. (1960)Experientia 16, 521.
Neuman, A., Becquart, J., Gillier, H., Leroux, Y., Queval, P., and Moretti J. L. (1989)Acta Crystallogr. C 45, 1966.
North, A. C. T., Philips, D. C., and Mathews, F. (1968)Acta Crystallogr. A 24, 351.
Parthasarathy, R., Paul, B., and Korytnyk, W. (1976)J. Am. Chem. Soc. 98, 6634.
Previtera, T., Basile, M., Vigorita, M. G., Fenech, G. Occhiuto, F., Circosta, C., and Costa De Pasquale, R. (1987)Eur. J. Med. Chem. 22, 67.
Scherrer, R. A. (1974) InAnti-inflammatory Agents Chemistry and Pharmacology, Scherrer, R. A., Whitehouse, M. N. (eds.), Vol. I, (Academic Press, New York), p. 29.
Sheldrick, G. M. (1976).Shelx 76.Program for Crystal Structure Determination. (Univ. of Cambridge, England).
Shen, T. H. (1967) InTopics in Medicinal Chemistry. Rabinowitz, J. L., and Meyerson, R. M. (eds.), (Wiley, New York), Vol. I, p. 29.
Vigorita, M. G., Previtera, T., Basile, M., Fenech, G., Costa De Pasquale, R., Occhiuto, F., and Circosta, C. (1984)11 Farmaco Ed. Sci. 39, 1008.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benetollo, F., Bombieri, G., Del Pra, A. et al. Structures of the configurational isomers of 3,3′ (1,2-ethanediyl) bis[2(3-fluorophenyl)-1,3-thiazolidine-4-one], C20H18F2N2O2S2 . Journal of Crystallographic and Spectroscopic Research 21, 113–120 (1991). https://doi.org/10.1007/BF01161051
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01161051