Abstract
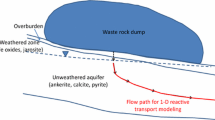
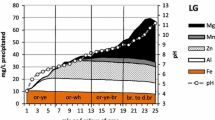
Solute transport and chemical neutralization (pH 3 to 7) within a shallow heterogeneous aquifer producing acid mine drainage (AMD) are examined at an abandoned surface coal mine in West Virginia. The aquifer is undergoing partial neutralization by mixing with alkalinity from a leaking sludge disposal pond, extending in preferential zones controlled by aquifer heterogeneity. Hydraulic heads interpolated from wells indicate leakage from a central alkaline (pH 7.1, 0.72 meq/L alkalinity) sludge pond is a principal source of recharge. Chemically-conservative sodium, added to AMD during treatment and leaked into the aquifer with the sludge, develops a dispersion plume over a restricted portion of the aquifer that correlates with pH, hydraulic head, and dissolved metals distributions. Concentrations of aluminum, iron, sulfate and acidity display higher concentrations downgradient from the pond as sludge alkalinity is consumed along flow paths. Before reaching springs, most dissolved iron is oxidized and hydrolyzed, likely precipitating in the aquifer as a ferric hydroxide or hydroxysulfate phase. The spatial pattern of iron and aluminum concentrations suggests accelerated oxidation caused by gas transport along the outer slopes of the spoil. Dissolved aluminum concentrations increase with total acidity, suggesting that dissolution of silicate minerals results from acidity released by iron hydrolysis. Neutralization reactions and higher pH are favored in more highly permeable portions of the spoil, where ferrihydrite and aluminum hydroxysulfate minerals (such as basaluminite) are supersaturated. In acid-producing zones at pH < 4.5, jurbanite is near equilibrium and an aluminum-sulfate phase with similar properties may limit aluminum concentrations, but become undersaturated in zones of advancing neutralization. At this particular site, ferrous iron produced by pyrite oxidation is almost completely oxidized over short transport distances, allowing hydrolysis of iron and aluminum should sufficient alkalinity be added to these acid waters.
Similar content being viewed by others
References
Alpers, C. N., Blowes, D. W., Nordstrom, D. K., and Jambor, J. L. (1994) Secondary minerals and acid mine-water chemistry, (eds. J. L. Jambor and D. W. Blowes),Environmental Geochemistry Of Sulfide Mine-Wastes. Mineralogical Association of Canada, Short Course Handbook Vol. 22: p. 247–270.
Alpers, C. N. and Nordstrom, D. K. (1991) Geochemical evolution of extremely acid mine waters at Iron Mountain, California: are there any lower limits to pH? inProceedings, Second International Conference on the Abatement of Acid Drainage,Vol. 2: pp. 321–342.
Ball, J. W. and Nordstrom, D. K. (1991) User's manual for WATEQ4F, with revised thermodynamic data base and test cases for calculating speciation of major, trace, and redox elements in natural waters. U.S. Geological Survey Open-File Report 91-0183, 193 pp.
Bigham, J. M. (1994) Mineralogy of ochre deposits formed by sulfide oxidation, (eds. J. L. Jambor and D. W. Blowes),Environmental Geochemistry Of Sulfide Mine-Wastes. Mineralogical Association of Canada, Short Course Handbook Vol. 22: pp. 103–132.
Bigham, J. M., Carlson, L., and Murad, E. (1994) Schwertmannite, a new iron oxyhydroxtsulphate from Pyhasalmi, Finland, and other localities.Mineralogical Magazine,Vol. 58: pp. 641–648.
Bigham, J. M., Schwertmann, U., Traina, S. J., Winland, R. L., and Wolf, M. (1996) Schwertmannite and the chemical modeling of iron in acid sulfate waters.Geochim. et Cosmochim. Acta 60, 2111–2121.
Blowes, D. W. and Ptacek, C. J. (1994) Acid neutralization mechanisms in inactive mine tailings. (eds. J. L. Jambor and D. W. Blowes),Environmental Geochemistry Of Sulfide Mine-Wastes. Mineralogical Association of Canada, Short Course Handbook Vol. 22, p. 271–292.
Caruccio, F. T., Geidel, G., and Williams, R. (1984) Induced alkaline recharge zones to mitigate acidic seeps. inSymposium on Surface Mining Hydrology, Sedimentology, and Reclamation, Lexington KY, pp. 94–103.
Cathles, L. M. and Apps, J. A. (1975) A model of the dump leaching process that incorporates oxygen balance, heat balance, and air convection.Metall. Trans. B,6B, 617–623.
Chapman, B. M., Jones, D. R., and Jung, R. F. (1983) Processes controlling metal ion attenuation in acid mine drainage streams.Geochim. et Cosmochim. Acta 47, 1957–1973.
Diodato, D. M. and Parizek, R.: (1994) Unsaturated hydrogeologic properties of reclaimed coal strip mines.Ground Water 32, 108–118.
Fillipek, L. H., Nordstrom, D. K., and Ficklin, W. H. (1987) Interaction of acid mine drainage with waters and sediments of West Squaw Creek in the West Shasta Mining District, California.Environ. Sci. Technol. 21, 388–396.
Frysinger, K. W.: 1996, Large-scale hydraulic characteristics of a heterogeneous Appalachian minespoil aquifer. Unpublished M.S. thesis, West Virginia University, 143 pp.
Guo, W., Parizek, R. R., and Rose, A. (1994) The role of thermal convection in resupplying O2 to strip coal-mine spoil.Soil Science 158, 47–55.
Hantush, M. S. (1967) Growth and decay of groundwater mounds in response to uniform percolation.Water Resour. Res. 3, 227–234.
Hawkins, J. W. and Aljoe, W. W.: (1992) Pseudokarst groundwater hydrologic characteristics of a mine spoil aquifer.Mine Water Environ. 11, 37–52.
Jambor, J. L. (1994) Mineralogy of sulfide-rich tailings and their oxidation products. (eds. J. L. Jambor and D. W. Blowes),Environmental Geochemistry Of Sulfide Mine-Wastes. Mineralogical Association of Canada, Short Course Handbook Vol. 22, pp. 59–102.
Karathanasis, A. D., Evangelou, V. P., and Thompson, Y. (1988) Aluminum and iron equilibria in soil solutions and surface waters of acid mine watersheds.J. Environ. Quality 17, 534–543.
Kittrick, J. A. (1966) The free energy of formation of gibbsite and Al(OH)4 − from solubility measurements.Soil Sci. Soc. Am. Proc. 30, 595–598.
Langmuir, D. and Melchior, D. (1985) The geochemistry of Ca, Sr, Ba, and Ra sulfates in some deep brines from the Palo Duro basin, Texas.Geochim. Cosmochim. Acta 49, 2423–2432.
Lindsay, W. A. (1979) The Chemistry of Soils. Wiley and Sons, New York, 359 pp.
May, H. M., Helmke, P. A., and Jackson, M. L. (1979) Gibbsite solubility and thermodynamic properties of hydroxy-aluminum ions in aqueous solution at 25°C.Geochim. Cosmochim. Acta 46, 681–692.
McKnight, D. M. and Bencala, K. 1989, Reactive iron transport in an acidic mountain stream in Summit County, Colorado; a hydrologic perspective.Geochim. Cosmochim. Acta 53, 2225–2234.
Monterroso, C., Alvarez, E., and Macias, A. (1994) Speciation and solubility control of Al and Fe in minesoil solutions.Sci. Total Environ. 158, 31–43.
Nawrot, J. R., Conley, P. S., and Sandusky, J. E. (1994) Concentrated alkaline recharge pools for acid seep abatement: principles, design, construction, performance.U.S. Bureau of Mines Special Publication SP 06A-94,Vol. 1, pp. 382–391.
Nicholson, R. V., Gillham, R. W., and Reardon, E. (1990) Pyrite oxidation in carbonate-buffered solution; 2, Rate control by oxide coatings.Geochim. Cosmochim. Acta 54, 395–402.
Nordstrom, D. K. (1982) The effect of sulfate on aluminum concentrations in natural waters: some stability relations in the system Al2O3-SO3-H2O at 298 ‡ K.Geochim. Cosmochim. Acta 46 681–692.
Nordstrom, D. K. and Alpers, C. J. (1997) (in press) Geochemistry of acid mine waters. (eds. G. S. Plumlee and M. J. Logson), Reviews in Economic Geology, Vol. 6,The Environmental Geochemistry of Mineral Deposits.
Nordstrom, D. K. and Ball, J. W. (1986) The geochemical behavior of aluminum in acidified surface waters.Science 232, 54–56.
Nordstrom, D. K., Plummer, L. N., Langmuir, D., Busenburg, E., May, H. M., Jones, B. F., and Parkhurst, D. L. (1990) Revised chemical equilibrium data for major water-mineral reactions and their limitations. (eds D. C. Melchior and R. L. Bassett),Chemical Modeling of Aqueous Systems II: American Chemical Society Symposium Series 416, p. 389–413.
Norvell, W. A. and Lindsay, W. L. (1982) Estimation of the concentration of Fe3+ and the Fe(OH)3 ion product from equilibria EDTA in soil.Soil Sci. Soc. Am. J. 46, 710–715.
Olson, G. J. (1991) Rate of bioleaching byThiobacillus ferroxidans: results of an interlaboratory comparison.Appl. Environ. Microbiol. 57, 642–644.
Renton, J. J., Stiller, A. H., and Rymer, T. (1989b) The acid-producing potential of the various rock units associated with the mining of coal.Mining Sci. Technol. 9, 137–147.
Ritchie, A. I. (1994) The waste-rock environment. (eds. J. L. Jambor and D. W. Blowes),Environmental Geochemistry Of Sulfide Mine-Wastes. Mineralogical Association of Canada, Short Course Handbook Vol. 22, pp. 133–162.
Sasowsky, I. D. and White, W. (1993) Geochemistry of the Obey River basin, north-central Tennessee; a case of acid mine water in a karst drainage system.J. Hydrology 146, 29–48.
Schwartz, F. W. and Crowe, A. S. (1985) Simulation of changes in groundwater levels associated with strip mining.Geol. Soc. Am. Bull. 96, 253–262.
Singer, P. C., and Stumm, W.: 1970, Acid mine drainage: the rate limiting step.Science 167, 1121–1123.
Snyder, D. T. and Caruccio, F. T. (1988) The partitioning of flow components of acidic seeps from surface coal mines and the identification of acid-producing horizons within the backfill, in1988 Mine Drainage and Surface Reclamation Conference, Pittsburgh, PA, pp. 59–66.
Sullivan, P. J., Yelton, J. L., and Reddy, K. (1988) Solubility relationships of aluminum and iron minerals associated with acid mine drainage.Environ. Geol. Water Sci. 11, 283–287.
Weiss, J. S. and Razem, A. C. (1984) Simulation of ground-water flow in a mined watershed in eastern Ohio.Ground Water 22, 549–560.
Wicks, C. M. and Groves, C. (1993) Acidic mine drainage in carbonate terrains; geochemical processes and rates of calcite dissolution.J. Hydrol. 146, 13–27.
Williamson, M. A. and Rimstidt, J. D. (1994) The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation.Geochim. Cosmochim. Acta 58, 5443–5454.
Wunsch, D. R. and Dinger, J. S. (1994) The hydrogeology and hydrogeochemistry of the Star Fire site, eastern Kentucky. U.S. Bureau of Mines Special Publication SP 06B-94, Vol. 2, pp. 188–197.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Donovan, J.J., Frysinger, K.W. & Maher, T.P. Geochemical response of acid groundwater to neutralization by alkaline recharge. Aquat Geochem 2, 227–253 (1996). https://doi.org/10.1007/BF01160044
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01160044