Abstract
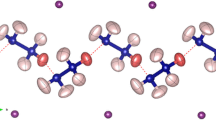
The X-ray crystal structure of 3-ammonium-4-hydroxyphenylarsonic acid chloride dihydrate has been determined from single-crystal diffraction data. The compound crystallizes in the orthorhombic space groupPna 21 with four molecules in a unit cell of dimensionsa=17.712(7),b=13.468(4), andc=4.798(2) Å. The structure was solved by the Patterson method and refined to a finalR value of 2.1%. The average C-C phenyl distance is 1.393 Å, but two bonds are somewhat shorter than the others. This, coupled with the fact that the C-O and C-As bonds are shorter than normal, makes it appear as if there is a minor resonance contributor of a keto form. The C-N bond length of 1.443 Å is intermediate between values found in aminophenols and other ammoniumphenols. The H2AsO3 group is nearly tetrahedral, with the double-bonded oxygen rotated 9° out of the phenyl plane about the C-As bond. There is an extensive hydrogen-bonding system, involving every one of the OH and NH hydrogens, through the chloride and the waters of crystallization.
Similar content being viewed by others
References
Avens, L. R. (1982) Ph.D. dissertation, Texas Tech University, Lubbock, Texas.
Bacon, G., and Jude, R. J. (1973)Z. Kristallogr. 138, 19.
Bernal, I., and Korp, J. D. (1983)Acta Cryst., manuscript in preparation.
Bijvoet, J. M., Peerdeman, A. F., and Van Bommel, A. J. (1951)Nature (London) 168, 271.
Bois, C. (1972)Acta Cryst. B 28, 25.
Brown, C. J. (1951)Acta Cryst. 4, 100.
Cesur, A. F., and Richards, J. P. G. (1965)Z. Kristallogr. 122, 283.
Chatterjee, A., and Sen Gupta, S. P. (1977a)Acta Cryst. B 33, 3593.
Chatterjee, A., and Sen Gupta, S. P. (1977b)Acta Cryst. B 33, 164.
Christiansen, W. G. (1920)J. Am. Chem. Soc. 42, 2402.
Cromer, D. T., and Liberman, D. J. (1970)J. Chem. Phys. 53, 1891.
Cromer, D. T., and Mann, J. B. (1968)Acta Cryst. A 24, 321.
de Rango, C., Brunie, S., and Tsoucaris, G. (1974)Cryst. Struct. Commun. 3, 485.
Ehrlich, P. (1907)Lancet 173, 351.
Herbstein, F. H., Kapon, M., Maor, I., and Reisner, G. M. (1981)Acta Cryst. B 37, 136.
Holmes, R. R. (1982) 183rd National American Chemical Society Meeting, Las Vegas, Nevada.
Korp, J. D., Bernal, I., Avens, L. R., and Mills, J. L. (1981)J. Cryst. Mol. Struct. 11, 117.
Kraft, M. Y., and Agracheva, E. B. (1955)Dokl. Akad. Nauk. SSSR 100, 279.
Kraft, M. Y., and Baskchuck, I. A. (1949)Dokl. Akad. Nauk SSSR 65, 509.
Kraft, M. Y., Bordina, G. M., Strel'tsova, I. N., and Struchkov, Y. T. (1960)Dokl. Akad. Nauk SSSR 131, 1074.
Kwak, W., Rajkovic, L. M., Stalick, J. K., Pope, M. T., and Quicksall, C. O. (1976)Inorg. Chem. 15, 2778.
Magill, L. S., Korp, J. D., and Bernal, I. (1981)Inorg. Chem. 20, 1187.
Shimada, A. (1960)Bull. Chem. Soc. Jpn. 33, 301.
Skapski, A. C., and Stevenson, J. L. (1975)J. Chem. Soc. Perkin Trans. II, 1197.
Smith, L. R., and Mills, J. L. (1975)J. Organomet. Chem. 84, 1, and references therein.
Smith, M. R., Zingaro, R. A., and Meyers, E. A. (1971)J. Organomet. Chem. 27, 341.
Stalhandske, C. (1974)Acta Cryst. B 30, 1586.
Stewart, R. F., Davidson, E. R., and Simpson, W. T. (1965)J. Chem. Phys. 42, 3175.
Wallwork, S. C. (1962)Acta Cryst. 15, 758.
Whuler, A., Brouty, C., and Spinat, P. (1980)Acta Cryst. B 36, 1267.
Wunderlich, H., and Mootz, D. (1971)Acta Cryst. B 27, 1684.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korp, J.D., Bernal, I., Avens, L. et al. The X-ray crystal structure of 3-ammonium-4-hydroxyphenylarsonic acid chloride dihydrate. Journal of Crystallographic and Spectroscopic Research 13, 263–272 (1983). https://doi.org/10.1007/BF01158906
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01158906