Abstract
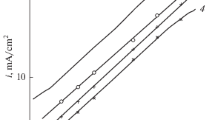
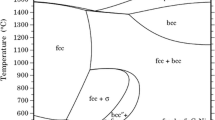
Alloys of composition (in weight percent) Fe-10Mn-10Cr, Fe-10Mn-25Cr, and Fe-25Mn-10Cr were reacted at temperatures of 973 and 1073 K with flowing hydrogen-hydrogen sulfide mixtures corresponding to equilibrium sulfur partial pressures of 10−3 and 8 Pa. Sulfide-scale-growth kinetics and morphologies were compared with those found on pure iron and on the binary alloys Fe-25Cr and Fe-25Mn. All alloys reacted according to parabolic kinetics after an initial period of slow approach to this steady state. Of the materials examined, the binary Fe-25Mn showed the slowest sulfidation rates, except at 973 K and a sulfur pressure of 8 Pa, where Fe-10Mn-25Cr had the best performance. Ternary alloys provided improved performance only when a scale layer of Cr3S4 was formed, an event dependent on temperature and sulfur activity. Multilayered scales were always formed on the ternary alloys, and the role of these layers in controlling sulfidation rates is discussed.
Similar content being viewed by others
References
D. J. Young,Rev. High Temp. Mater. 4, 299 (1980).
F. J. Bruns,Corrosion 17, 227 (1961).
F. J. Bruns,Corrosion 25, 119 (1969).
E. B. Backensto, R. D. Drew, and C. C. Stapleford,Corrosion 12, 6t (1956).
L. I. Staffanson,Met. Trans. B 7B, 131 (1976).
S. Mrowec and K. Przybylski,High Temp. Mater. Proc. 6, 1 (1984).
K. Nishida, T. Narita, T. Tani, and G. Sasaki,Oxid. Met. 14, 65 (1980).
K. Nishida and T. Narita,Proc. 8th Int. Cong. Met. Corros. 1, 821 (1981).
S. Mrowec, T. Walec, and T. Werber,Oxid. Met. 1, 93 (1969).
T. Narita and K. Nishida,Oxid. Met. 6, 157 (1973).
T. Narita, W. W. Smeltzer, and K. Nishida,Oxid. Met. 17, 299 (1982).
T. Narita and W. W. Smeltzer,Oxid. Met. 21, 39 (1984);21, 57 (1984).
P. Papaiacovou, H. J. Grabke, and P. Schmidt,Werkst. Korros. 36, 320 (1985).
D. de B. Darwent and R. Roberts,Proc. Roy. Soc., London Ser A. 216, 344 (1953
J. P. Orchard and D. J. Young,J. Electrochem. Soc. 133, 1734 (1986).
T. Rosenqvist,J. Iron Steel Inst. 174, 37–59 (1954).
K. T. Jacob, D. B. Rao, and H. G. Nelson,Oxid. Met. 13, 25–55 (1979).
D. J. Young, W. W. Smeltzer, and J. S. Kirkaldy,J. Electrochem. Soc. 120, 1221–1224 (1973).
H. Rau,J. Less Common Met. 55, 205–211 (1977).
O. Kubaschewski and C. B. Alcock,Metallurgical Thermochemistry, 5th Ed. (Perg Oxford, 1979), p. 381.
F. A. Elrefaie and W. W. Smeltzer,Oxid. Met. 16, 267–275 (1981).
R. Kiessling and C. Westman,J. Iron Steel Inst. 377–379 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Southwell, G., Young, D.J. High-temperature sulfidation of Fe-Mn-Cr alloys. Oxid Met 36, 409–421 (1991). https://doi.org/10.1007/BF01151589
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01151589