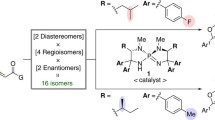
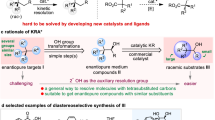
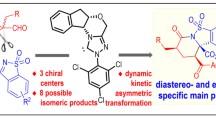
Abstract
Highly efficient kinetic resolution of racemic secondary alkyl diazoacetates in intramolecular carbon-hydrogen insertion reactions has been achieved using chiral dirhodium(ii) carboxamidates. Products formed from catalytic diazo decomposition of racemic 2-octyl diazoacetate and, separately, its (2R)- and (2S)-enantiomeric forms, as well as bothcis- andtrans-2-methylcyclohexyl diazoacetates, have been systematically evaluated. Enantioselectivities up to 99 %ee have been obtained for γ-lactone formation. β-Lactone production has been observed and, although minor with cyclohexyl diazoacetates, is the major insertion pathway for diazo decomposition of 2-octyl diazoacetate.
Similar content being viewed by others
References
M. P. Doyle,Izv. Akad. Nauk, Ser. Khim., 1994, 1770 [Russ. Chem. Bull., 1994,43, 1879 (Engl. Transl.)].
M. P. Doyle, A. B. Dyatkin, G. H. P. Roos, F. Canas, D. A. Pierson, A. van Basten, P. Muller, and P. Polleux,J. Am. Chem. Soc., 1994,116, 4507.
M. P. Doyle, A. B. Dyatkin, and J. S. Tedrow,Tetrahedron Lett., 1994,35, 3853.
P. Muller and P. Polleux,Helv. Chim. Acta, 1994,77, 645.
M. P. Doyle, W. R. Winchester, J. A. A. Hoorn, V. Lynch, S. H. Simonsen, and R. Ghosh,J. Am. Chem. Soc., 1993,115, 9968.
M. P. Doyle, A. B. Dyatkin, M. N. Protopopova, C. I. Yang, C. S. Miertschin, W. R. Winchester, S. H. Simonsen, V. Lynch, and R. Ghosh,Rec. Trav. Chim. Pays-Bas, 1995,114, 163.
M. A. McKervey and T. Ye,J. Chem. Soc., Chem. Commun., 1992, 823.
S. Hashimoto, N. Watanabe, T. Sato, M. Shiro, and S. Ikegami,Tetrahedron Lett., 1993,34, 5109.
M. P. Doyle and A. B. Dyatkin,J. Org. Chem., 1995,60, 3035.
J. H. Boyer, G. H. Mack, W. Goebel, and L. R. Morgan,J. Org. Chem., 1959,24, 1051.
Y. Yamamoto, Y. Chounan, S. Nishii, T. Ibuka, and H. Kitahara,J. Am. Chem. Soc., 1992,114, 7652.
T. Kunz, A. Janowitz, and H.-U. Reissig,Chem. Ber., 1989,122, 2165.
S. Byström, H.-E. Högberg, and T. Norin,Tetrahedron, 1981,37, 2249.
R. J. Clemens and J. A. Hyatt,J. Org. Chem., 1985,50, 243.
T. K. DasGupta, D. Felix, U. M. Kempe, and A. Eschenmoser,Helv. Chim. Acta, 1972,55, 2198.
Author information
Authors and Affiliations
Additional information
Dedicated to Academician of the RAS N. S. Zefirov (on his 60th birthday).
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1798–1803, September, 1995.
Financial support for this research from the National Institutes of Health (GM 46503) and the National Science Foundation of the United States is gratefully acknowledged. We thank D. A. Pierson for her preparation of 2-methylcyclohexyl diazoacetates and preliminary studies of their diazo decomposition and A. Melekhov from the Higher College of Chemistry for his preparation and catalytic studies of rac-2-octyl diazoacetate.
Rights and permissions
About this article
Cite this article
Doyle, M.P., Kalinin, A.V. Enantiomer differentiation in intramolecular carbon—hydrogen insertion reactions of racemic secondary alkyl diazoacetates catalyzed by chiral dirhodium(ii) carboxamidates. Russ Chem Bull 44, 1729–1734 (1995). https://doi.org/10.1007/BF01151300
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01151300