Abstract
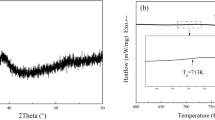
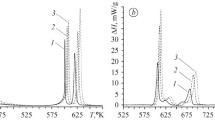
The amorphous to crystalline transition in Zr-33 at% Ni amorphous alloy has been found to occur by polymorphic crystallization. The product of the cyrstallization process has been identified as the equilibrium Zr2Ni intermetallic phase. The kinetics of crystallization have been studied independently using differential scanning calorimetry (DSC) and transmission electron microscopy. The activation energy of crystallization has been evaluated by isothermal and continuous heating in DSC. The isothermal anneals have revealed that the crystallization follows the Johnson-Mehl-Avrami kinetics with an Avrami exponent close to 4. The microstructural changes accompanying crystallization have been studied for an interpretation of the Avrami exponent. The nucleation and growth rates of crystals have been estimated at different temperatures in order to determine the activation energies of the two processes. It has been found that nucleation is thermally activated and growth is interface controlled.
Similar content being viewed by others
References
U. Koster andU. Herold, “Topics in Applied Physics”, Vol. 46 (Springer Verlag, New York, 1980) p. 225.
M. G. Scott, G. Gregan andY. D. Dong, Proceedings of the 4th International Conference on Rapidly Quenched Metals, Sendai, 1981, (Japan Institute of Metals, Sendai, 1982) p. 671.
U. Koster andU. Herold, Proceedings of the 4th International Conference on Rapidly Quenched Metals, Sendai, 1981 (Japan Institute of Metals, Sendai, 1982) p. 717.
H. E. Kissinger,Anal. Chem. 29 (1957) 1702.
J. Burke, The Kinetics of Phase Transformation in Metals and Alloys” (Pergamon Press, Oxford, 1965) p. 433.
J. C. Gachon, M. Dirand andJ. Hertz,J. Less-Common Metals 92 (1983) 307.
M. E. Fine, “Introduction to Phase Transformations in Condensed Systems” (MacMillan, London, 1964) p. 14.
J. W. Christian, “The Theory of Transformations in Metals and Alloys” (Pergamon Press, Oxford, 1965) p. 433.
Y. D. Dong, G. Gregan andM. G. Scott,J. Non-Cryst. Solids 43 (1981) 403.
G. K. Dey andS. Banerjee, Proceedings of 5th International Conference on Rapidly Quenched Metals, Wurzburg, 1984 (Elsevier, Amsterdam, 1985) p. 345.
M. G. Scott andP. Ramachandra Rao,Mater. Sci. Eng. 29 (1977) 137.
E. G. Baburaj, G. K. Dey, M. J. Patni andR. Krishnan,Scripta Metall. 19 (1985) 305.
U. Koster, “Phase Transformations in Crystalline and Amorphous Alloys”, edited by B. L. Mordike (Deutsche Gasellschaft fur Metallkunde, Oberussel, 1983) p. 113.
S. Ranganathan, J. C. Claus, R. S. Tiwari andM. V. Heimendahl, Proceedings of Conference on Metallic Glasses: Science and Technology, Budapest, 1980, Vol. I, p. 327.
S. Ranganathan andM. Von Heimendahl,J. Mater. Sci. 16 (1981) 2401.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dey, G.K., Baburaj, E.G. & Banerjee, S. Crystallization kinetics of Zr-33 at % Ni amorphous alloy. J Mater Sci 21, 117–122 (1986). https://doi.org/10.1007/BF01144708
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01144708