Summary
-
1.
From the unhydrolyzable part of the seed oil ofSchizandra chinensis two new substances were isolated: deoxyschizandrin and pseudo-γ-schizandrin.
-
2.
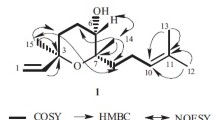
The structures of deoxyschizandrin andγ-schizandrin were established; they belong to a new group of natural substances derived from 5,6,7,8-tetrahydrodibenzo[a, c] cyclooctene. The structure of deoxyschizandrin was confirmed by the total synthesis of its racemate.
-
3.
The stereochemistry of deoxyschizandrin, γ-schizandrin, and pseudo-γ-schizandrin is discussed.
Similar content being viewed by others
Literature cited
N. K. Kochetkov, A. Ya. Khorlin, and O. S. Chizhov, Zh. obshch. khimii31, 3554 (1961).
N. K. Kochetkov, A. Ya. Khorlin, O. S. Chizhov, and V. I. Sheichenko, Tetrahedron Letters, No. 20, 730 (1961).
N. K. Kochetkov, A. Ya. Khorlin, O. S. Chizhov, and V. I. Sheichenko, Izv. AN SSSR. Otd. khim. n.,1962, 850.
N. K. Kochetkov, A. Ya, Khorlin, and O. S. Chizhov, Izv. AN SSSR. Otd. khim. n.1962, 856.
I. R. Bick, J. Harley-Mason, H. Sheppard, and J. Vernengo, J. Chem. Soc.1961, 1896.
E. Späth and H. Quietensky, Ber.60, 1882 (1927).
S. V. Lieberman, G. P. Mueller, and E. T. Stiller, J. Amer. Chem. Soc.69, 1540 (1947).
T. Kaku and H. Ri, J. Pharm. Soc. Japan57, 289 (1937); S. Keimatsu and T. Ishiguro, J. Pharm. Soc. Japan,56, 61 (1936); G. K. Hughes and E. Ritchie, Austral. J. Chem.7, 104 (1954); A. J. Birch, G. K. Hughes, and E. Smith, Austral. J. Chem.7, 83 (1954).
A. W. Schrecker and J. L. Hartwell, J. Amer. Chem. Soc.77, 432 (1955).
L. V. Dvorken, R. B. Smith, and K. Mislow, J. Amer. Chem. Soc.80, 486 (1958).
L. A. Wiles, Chem. Rev.56, 329 (1956).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Setiya Khimicheskaya, No. 6, pp. 1036–1042, June, 1964
Rights and permissions
About this article
Cite this article
Kochetkov, N.K., Khorlin, A.Y. & Chizhov, O.S. Chemical investigation of schizandra chinensis Communication 4. Isolation, structure, and synthesis of deoxyschizandrin, and the structure of γ-schizandrin. Russ Chem Bull 13, 963–968 (1964). https://doi.org/10.1007/BF01141648
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01141648