Summary
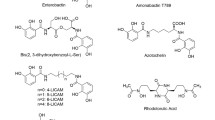
Linear hydroxamate derivatives, possessing chiral α-amino acid moieties, were synthesized and their iron transport activities were studied in bacteria and fungi. No growth-promoting activity could be detected in the Gram-positive hydroxamate-auxotrophAureobacterium flavescens JG9. However, Gram-negative enterobacteria, such asEscherichia coli, Pantoea agglomerans andHafnia alvei were able to utilize iron from these analogues. Uptake of55Fe-labeled analogues was inhibited by sodium azide, suggesting an active transport process. The receptors involved during uptake in enterobacteria were identified by using appropriate indicator organisms which are defective in the transport of either ferrioxamines (P. agglomerans FM13), coprogens (H. alvei), or both of these siderophore classes (E. coli fhuE). Our data suggest that the chiral hydroxamates are recognized by the ferrioxamine receptor (FoxA) and the coprogen receptor (FhuE) at a ratio which depends on the opticalν/δ isomer fraction and the nature of side chains. Transport was also observed in the fungusNeurospora crassa, known to take up coprogen rather than ferrioxamines, suggesting that in this fungus the synthetic analogues behave like coprogen.
Similar content being viewed by others
References
Berner I, Winkelmann G (1990) Ferrioxamine transport mutants and the identification of the ferrioxamine receptor protein (FoxA) inErwinia herbicola (Enterobacter agglomerans). Biol Metals 2:197–202
Berner I, Konetschny-Rapp S, Jung G, Winkelmann G (1988) Characterization of ferrioxamine E as the principal siderophore ofErwinia herbicola (Enterobacter agglomerans). Biol Metals 1:51–56
Bickel H, Bosshardt R, Gäumann E, Reusser P, Vischer E, Voser W, Wettstein A, Zähner H (1960) Stoffwechselprodukte von Actinomyceten. 26. Mitteilung. Über die Isolierung und Charakterisierung der Ferrioxamine A-F, neuer Wuchsstoffe der Sideramine-Gruppe. Helv Chim Acta 43:2105–2118
Braun K, Gunter K, Hantke K (1991) Transport of iron across the outer membrane. Biol Metals 4:14–22
Emery T, Emery L, Olson RK (1984) Retrohydroxamate ferrichrome: A biomimetic analogue of ferrichrome. Biochem Biophys Res Commun 119:1191–1197
Gavini F, Mergaert J, Beji A, Mielcarek C, Izard D, Kersters K, De Ley J (1989) Transfer ofEnterobacter agglomerans (Beijerinck 1888) Ewing and Five 1972 toPantoea gen nov. asPantoea agglomerans comb. nov. and description ofPantoea dispersa sp. nov. Int J Syst Bacteriol 39:337–345
Hantke K (1983) Identification of an iron uptake system specific for coprogen and rhodotorulic acid inEscherichia coli K12. Mol Gen Genet 191:301–306
Huschka H, Winkelmann G (1989) Iron limitation and its effect on membrane proteins and siderophore transport inNeurospora crassa. Biol Metals 2:108–113
Konetschny-Rapp S, Huschka H, Winkelmann G, Jung G (1988a) High-performance liquid chromatography of siderophores from fungi. Biol Metals 1:9–17
Konetschny-Rapp S, Jung G, Huschka H, Winkelmann G (1988b) Isolation and identification of the principal siderophore of the plant pathogenic fungusBotrytis cinerea. Biol Metals 1:90–98
Köster W (1991) Iron(III) hydroxamate transport across the cytoplasmic membrane ofEscherichia coli. Biol Metals 4:23–32
Meyer JM, Abdallah MA (1980) The siderochromes of nonfluorescent pseudomonads: production of nocardamine byPseudomonas stutzeri. J Gen Microbiol 118:125–129
Reissbrot R, Rabsch W, Chapeaurouge A, Jung G, Winkelmann G (1990) Isolation and identification of ferrioxamines G and E inHafnia alvei. Biol Metals 3:54–60
Sauer M, Hantke K, Braun V (1987) Ferric coprogen receptor FhuE ofEscherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J Bacteriol 169:2044–2049
Shanzer A, Libman J (1989) Synthetic siderophores as biological probes. Biol Metals 2:129–134
Shanzer A, Libman J, Lazar R, Tor Y, Emery T (1988) Synthetic ferrichrome analogues with growth promotion activity forArthrobacter flavescens. Biochem Biophys Res Commun 157:389–394
van der Helm D, Jalal MAF, Hossain MB (1987) The crystal structures, conformations and configurations of siderophores. In: Winkelmann G, van der Helm D, Neilands JB (eds) Iron transport in microbes, plants and animals, VCH Verlagsgesellschaft, Weinheim pp 135–165
Winkelmann G, Zähner H (1973) Stoffwechselprodukte von Mikroorganismen. 115. Mitteilung. Eisenaufnahme beiNeurospora crassa. I. Zur Spezifität des Eisentransportes. Arch Microbiol 88:49–60
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berner, I., Yakirevitch, P., Libman, J. et al. Chiral linear hydroxamates as biomimetic analogues of ferrioxamine and coprogen and their use in probing siderophore-receptor specificity in bacteria and fungi. Biol Metals 4, 186–191 (1991). https://doi.org/10.1007/BF01141313
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01141313