Summary
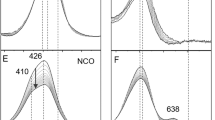
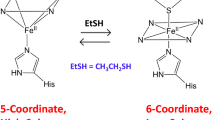
Binding of azide to type-2-copper-depleted (T2D) zucchini ascorbate oxidase, containing reduced type-3 Cu centers, and met-T2D ascorbate oxidase, containing oxidized type-3 Cu centers, has been studied spectroscopically. In both cases titration with azide in 0.1 M phosphate pH 6.8 produces a broad near-ultraviolet band with maximum at 455 nm (Δe ≈ 2500 M−1 cm−1, with respect to the met-T2D enzyme) and shoulder at 390 nm (Δe ≈ 1700 M−1 cm−1), that are assigned toπ(azide)→Cu(II) ligand-to-metal charge transfer (LMCT) transitions. This is accompanied by a reduction of absorbance at 330 nm in the met-T2D) enzyme adduct (Δe ≈ −1400 M−1 cm−1). A broad circular dichroic band of negative sign between 370–480 nm corresponds to the LMCT absorption band. Analysis of the titration data indicates that one azide ion binds independently to each of the binuclear T3 Cu couples with low affinity (K = 50 M−1). The ESR signal of the T1 Cu observed in frozen solutions of the T2D enzyme is also perturbed by the addition of azide. The analogies in the azide-binding characteristics between ascorbate oxidase and laccase are discussed.
Similar content being viewed by others
References
Agnus Y, Louis R, Gisselbrecht J-P, Weiss R (1984) Dicopper(II) chloro and azido inclusion complexes of the [24-ane-N2S4] binucleating macrocycle. Synthesis, crystal and molecular structures, and spectral magnetic and electrochemical properties. J Am Chem Soc 106:93–102
Aikazyan V Ts, Nalbandyan RM (1979) Copper-containing proteins fromCucumis sativus. FEBS Lett 104:127–130
Allendorf MD, Spira DJ, Solomon EI (1985) Low-temperature magnetic circular dichroism studies of native laccase: spectroscopic evidence for exogenous ligand bridging at a trinuclear copper active site. Proc Natl Acad Sci USA 82:3063–3067
Antonini E, Brunori M (1971) The equilibrium of hemoglobin and myoglobin with ligands. In: Antonini E, Brunori M (eds) Hemoglobin and myoglobin in their reactions with ligands. North-Holland, Amsterdam, pp 153–187
Avigliano L, Desideri A, Urbanelli S, Mondovi B, Marchesini A (1979) Removal of non-blue copper from ascorbate oxidase. FEBS Lett 100:318–320
Avigliano L, Vecchini P, Sirianni P, Marcozzi G, Marchesini A, Mondovi B (1983) A reinvestigation on the quaternary structure of ascorbate oxidase fromCucurbita pepo medullosa. Mol Cell Biochem 56:107–112
Bernaducci EE, Schwindinger WF, Hughey IV JL, Krogh-Jespersen K, Schugar HJ (1981) Electronic spectra of copper(II)-imidazole and copper(II)-pyrazole chromophores. J Am Chem Soc 103:1686–1691
Casella L, Gullotti M (1983) Coordination modes of histidine. Copper(II) complexes ofl-N τ-methylhistidine andl-N α,N α-dimethylhistidine in aqueous solution. Inorg Chem 22:242–249
Casella L, Fantucci P, Gullotti M, Marchesini A (1984) Spectral study of ascorbate oxidase. Inorg Chim Acta 91:189–194
Casella L, Gullotti M, Pallanza G, Pintar A, Marchesini A (1988a) Azide-binding studies reveal type-3 copper heterogeneity in ascorbate oxidase from the green zucchini squash. Biochem J 251:441–446
Casella L, Gullotti M, Pallanza G, Rigoni L (1988b) Synthesis and reactivity of a family of copper monooxygenase model system. J Am Chem Soc 110:4221–4227
Casella L, Gullotti M, Pintar A, Pallanza G, Marchesini A (1989) Investigation of azide binding to oxidized type-2-copper-depleted ascorbate oxidase fromCucurbita pepo. J Inorg Biochem 37:105–109
Casella L, Gullotti M, Pallanza G, Buga M (1990) Binding of azide and thiocyanate ligands to copper(II) model complexes. Biol Metals 3:137–140
Dawson CR (1966) Ascorbate oxidase, a review. In: Peisach J, Aisen P, Blumberg WE (eds) The biochemistry of copper. Academic Press, New York, pp 305–337
Deinum J, Reinhammar B, Marchesini A (1974) The stoichiometry of the three different types of copper in ascorbate oxidase from green zucchini squash. FEBS Lett 42:241–245
Desjardins SR, Wilcox DE, Musselman RL, Solomon EI (1987) Polarized single-crystal, electronic spectral studies of Cu2Cl 2−6 : excited-state effects of the binuclear interaction. Inorg Chem 26:288–300
Fawcett TG, Bernaducci EE, Krogh-Jespersen K, Schugar HJ (1980) Charge-transfer absorptions of Cu(II)-imidazole and Cu(II)-imidazolate chromophores. J Am Chem Soc 102:2598–2604
Fee JA (1975) Copper proteins. Systems containing the ‘blue’ copper center. Struct Bonding 23:1–60
Frank P, Pecht I (1986) Redox titration of type-2-copper-depletedRhus laccase: reductive decoupling and oxidative reconstitution of the type-3 site. J Phys Chem 90:3809–3814
Hanna PM, McMillin DR, Pasenkiewicz-Gierula M, Antholine WE, Reinhammar B (1988) Tye-2-depleted fungal laccase. Biochem J 253:561–568
Karlin KD, Farooq A, Hayes JC, Cohen BI, Rowe TM, Sinn E, Zubieta J (1987) Models for met-hemocyanin derivatives: structural and spectroscopic comparisons of analogous phenolate and X (X=OH−, OMe−, N3, Cl−, OAc−, OBz−) doubly bridged dinuclear copper(II) complexes. Inorg Chem 26:1271–1280
Kau L-S, Spira-Solomon DJ, Penner-Hahn JE, Hodgson KO, Solomon EI (1987) X-ray absorption edge determination of the oxidation state and coordination number of copper: application to the type-3 site inRhus vernicifera laccase and its reaction with oxygen. J Am Chem Soc 109:6433–6442
Kawahara K, Suzuki S, Sakurai T, Nakahara A (1985) Characterization of cucumber ascorbate oxidase and its reaction with hexacyanoferrate(II). Arch Biochem Biophys 241:179–186
Kroneck PMH, Jakob W (1985) Ascorbate oxidase: further investigations of its structure and catalytic properties. Rev Port Quim 27:47–48
Kroneck PMH, Armstrong FA, Merkle H, Marchesini A (1982) Ascorbate oxidase: molecular properties and catalytic activity. Adv Chem Ser 200:223–248
LuBien CD, Winkler ME, Thamann TJ, Scott RA, Co MS, Hodgson KO, Solomon EI (1981) Chemical and spectroscopic properties of the binuclear copper active site inRhus laccase: direct confirmation of a reduced binuclear type-3 copper site in type-2-depleted laccase and intramolecular coupling of the type-3 to the tye-1 and type-2 copper sites. J Am Chem Soc 103:7014–7016
Marchesini A, Kroneck PMH (1979) Ascorbate oxidase fromCucurbita pepo medullosa. Eur J Biochem 101:65–76
McKee V, Zvagulis M, Dagdigian JV, Patch MG, Reed CA (1984) Hemocyanin models: synthesis, structure, and magnetic properties of a binucleating copper(II) system. J Am Chem Soc 106:4765–4772
Messerschmidt A, Rossi A, Ladenstein R, Huber R, Bolognesi M, Gatti G, Marchesini A, Petruzzelli R, Finazzi-Agrò A (1989) X-ray crystal structure of the blue ascorbate oxidase from zucchini. J Mol Biol 206:513–529
Morpurgo L, Graziani MT, Finazzi-Agrò A, Rotilio G, Mondovi B (1980) Optical properties of Japanese lacquer tree (Rhus vernicifera) laccase depleted of type-2 copper(II). Biochem J 187:361–366
Morpurgo L, Savini I, Mondovì B, Avigliano L (1987) Removal of type-2 Cu from ascorbate oxidase and laccase by reaction withN,N-diethyldithiocarbamate. J Inorg Biochem 28:25–31
Morpurgo L, Savini S, Gatti G, Bolognesi M, Avigliano L (1988) Reassessment of copper stoichiometry in ascorbate oxidase. Biochem Biophys Res Commun 152:623–628
Reinhammar B (1983a) Optical properties and a new epr signal from type-3 copper in metal-depletedRhus vernicifera laccase. J Inorg Biochem 18:113–121
Reinhammar B (1983b) Metal coordination and mechanism of blue-copper-containing oxidases. In: Bertini I, Drago RS, Luchinat C (eds) The coordination chemistry of metalloenzymes. D Reidel, Dordrecht, pp 177–200
Reinhammar B, Malmström BG (1981) ‘Blue’-copper-containing oxidases. In: Spiro TG (ed) Copper proteins. Wiley-Interscience, New York, pp 109–149
Ross PK, Allendorf MD, Solomon EI (1989) Detailed spectral studies of copper acetate: excited state interactions in copper dimers. J Am Chem Soc 111:4009–4021
Sakurai T, Sawada S, Suzuki S, Nakahara A (1985) Oxidation of reduced cucumber ascorbate oxidase. Biochem Biophys Res Commun 131:647–652
Sakurai T, Sawada S, Suzuki S, Nakahara A (1986) An investigation of reduction process of cucumber ascorbate oxidase. Biochem Biophys Res Commun 135:644–648
Sakurai T, Sawada S, Suzuki S, Nakahara A (1987) Properties of the type-II-copper-depleted cucumber ascorbate oxidase and its reaction with azide. Biochim Biophys Acta 915:238–245
Sakurai T, Suzuki S, Sano M (1988) X-ray absorption study on the type-II-copper-depleted cucumber ascorbate oxidase. Inorg Chim Acta 152:3–4
Solomon EI (1981) Binuclear copper active site. In: Spiro TG (ed) Copper proteins. Wiley-Interscience, New York, pp 41–108
Solomon EI, Penfield KP, Wilcox DE (1983) Active sites in copper proteins. An electronic structure overview. Struct Bonding 53:1–57
Sorrell TN, Borovik AS (1986) Luminescence behavior of copper(I)-imidazole complexes. A spectroscopic model for the carbonyl derivative of hemocyanin. J Am Chem Soc 108:2479–2481
Spira-Solomon DJ, Allendorf MD, Solomon EI (1986) Low-temperature magnetic circular dichroism studies of native lactase: confirmation of a Binuclear-copper active site. J Am Chem Soc 108:5318–5328
Spira-Solomon DJ, Solomon EI (1987) Chemical and spectroscopic studies of the coupled binuclear copper site in type-2-depletedRhus lactase: comparison to the hemocyanins and tyrosinase. J Am Chem Soc 109:6421–6432
Tyeklar Z, Karlin KD (1989) Copper-dioxygen chemistry: a bioinorganic challenge. Acc Chem Res 22:241–248
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casella, L., Gullotti, M., Pallanza, G. et al. Spectroscopic and binding studies of azide to type-2-copper-depleted ascorbate oxidase from zucchini. Biol Metals 4, 81–89 (1991). https://doi.org/10.1007/BF01135383
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01135383