Abstract
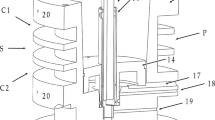
Using multiple bipolar electrolytic separation of hydrogen isotopes with Pd-25%Ag electrodes, the mathematical feasibility of this method for tritium separation was shown and experimentally verified. Separation factors were measured on single bipolar electrodes and were found to be approximately equivalent to those associated with individual ordinary electrolytic systems. Multibipolar separations were experimentally achieved in single cascaded cells in which each bipolar electrode was of equal area to others in a series arrangement. Factors measured for multibipolar H-D separation were close to the values measured in single-stage cell measurements; for H-T separation, interstage leakage reduced the measured separation factor. However, in both cases separation of sufficient magnitude was achieved to show feasibility for real application to the extraction of tritium from large-volume systems at high current density.
Similar content being viewed by others
References
H. K. Rae (editor), ‘Separation of Hydrogen Isotopes’, American Chemical Society Symposium Series 68, Washington DC (1978).
O. N. Salmon, ‘The Electrolytic Separation of Hydrogen Isotopes by Migration Through a Palladium Membrane’,Report KAPL-1272, Knolls Atomic Power Laboratory (General Electric Co.), Schenectady, New York, (June 25 1956).
F. T. Barr and W. P. Drews,Chem. Eng. Prog. 56 (1960) 49.
D. M. Drazic, Progress Report to EPA from the Faculty of Technology, University of Belgrade, Belgrade, Yugoslavia (1973).
S. V. Ribnikar and J. D. Pupezin, ‘Possibilities of Tritium Removal from Waste Waters of Pressurized Water Reactors and Fuel Reprocessing Plants’, presented at the 13th AEC Air Cleaning Conference, San Francisco, California (1974).
F. B. Longtin, private communication.
D. W. Ramey, M. Petek, R. D. Taylor, E. H. Kobisk, J. O. Ramey and C. A. Sampson,Report ORNL-5581, Oak Ridge National Laboratory, Tennessee (1979).
D. W. Ramey, M. Petek and E. H. Kobisk,Proc. Symp. Waste Management and Fuel Cycles, Tucson Arizona (edited by R. G. Post) (1978) p. 306.
J. Bigeleisen,Proc. Int. Symp. IAEA, Vol. I (1961) p. 161.
L. P. Roy,Can. J. Chem. 40 (1962) 1452.
S. Kaufman and W. F. Libby,Phys. Rev. 93 (1954) 1337.
B. Dandapani and M. Fleischmann,J. Electroanal. Chem. 39 (1972) 323.
H. Brodowsky, H. Gibmeyer and E. Wicke,Z Phys. Chem. N.F. 49 (1966) 222.
J. B. Hunter,Amer. Chem. Soc., Div. Petrol. Chem. 8 (1963) B49.
E. T. Serfass, ‘Activated Surfaces Useful in the Production of Hydrogen’, US Patent 3448 035 (1966).
M. Fleischmann and J. N. Hiddleston,J. Sci. Instrum. (Series 2)1 (1968) 667.
H. Brodowsky and E. Poeschel,Z. Phys. Chem. N.F. 44 (1965) 143.
A. Kussner,ibid 36 (1963) 383.
E. Wicke and G. Holleck,ibid 46 (1965) 123.
E. Wicke and H. Brodowsky, ‘Topics in Applied Physics: Hydrogen in Metals II’, Vol. 29, Springer-Verlag, Berlin (1978) p. 73.
H. Brodowsky and E. Wicke,Technical Bulletin, Engelhard Industries Inc., Menlo Park, New Jersey7 (1966) 41.
B. E. Conway,Proc. Roy. Soc. A256 (1960) 128.
J. O'M Bockris and S. Srinivasan,J. Electrochem. Soc. 111 (1964) 844.
Idem, ibid 111 (1964) 853.
Idem, ibid 111 (1964) 858.
P. W. T. Lu and S. Srinivasan,J. Appl. Electrochem. 9 (1979) 269.
I. N. Maksimova and V. F. Yushkevich,Russian J. Phys. Chem. 37 (1963) 475.
H. R. C. Pratt, ‘Countercurrent Separation Processes’, Elsevier, Amsterdam (1967).
D. W. Ramey, M. Petek, R. D. Taylor, P. W. Fischer, E. H. Kobisk, J. Ramey and C. A. Sampson,Separation Sci. Technol. 15 (1980) 405.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Petek, M., Ramey, D.W. & Taylor, R.D. Tritium separation from light and heavy water by bipolar electrolysis. J Appl Electrochem 11, 477–488 (1981). https://doi.org/10.1007/BF01132436
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01132436