Summary
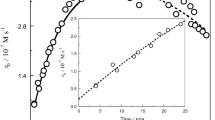
The kinetics of the oxidation of iodide by hexacyanoferrate(III) have been studied in different H2O-methanol, t-butanol, ethylene glycol glycerol, glucose or sucrose mixtures. Specific solvent effects are found as shown by the different trends in activation parameters and in the variations of the initial state and transition state chemical potentials as observed in different media. From the results of a multiple linear correlation, it is concluded that the kinetics are controlled by the ionizing power of the solvents, their relaxation times and the outer sphere reorganization energies.
Similar content being viewed by others
References
C. Reichardt,Angew. Chem. Int. Edit.,4, 29 (1965).
O. W. Kolling,J. Phys. Chem.,90, 4644 (1986).
M. J. Blandamer and J. Burgess,Coord. Chem. Rev.,31, 93 (1980).
A. E. Eid and C. F. Wells,J. Chem. Soc. Faraday Trans 1,79, 253 (1983).
P. Pérez Tejeda, J. Rodriguez Velasco and F. Sánchez Burgos,J. Chem. Soc. Dalton Trans., 2679 (1983).
M. J. Blandamer, J. Burgess and R. I. Haines,J. Chem. Soc. Dalton Trans., 1293 (1963).
E. Grunwald and S. Winstein,J. Am. Chem. Soc.,70, 846 (1948).
E. Muñoz, I. Tejera, R. Jiménez and F. Sánchez Burgos, React.Kinet. Catal. Lett., 1989.
A. Dogonadze, in N. S. Hush, (Ed.)Reactions of Molecules at Electrodes, Wiley Interscience, New York, 1971.
L. D. Zusman,Chem. Phys.,49, 295 (1980).
Handbook of Chemistry and Physics, C.R.C. Press 1972–73.
Handbook of Chemistry and Physics, C.R.C. Press 1977–78.
P. S. Yastremskii V. S. Khar'kin, V. S. Goncharov and A. K. Lyashchenko,Russ. J. Phys. Chem.,57, 49 (1983).
V. S. Khar'kin, P. S. Yastremskii and A. K. Lyashchenko,Russ. J. Phys. Chem.,56, 1240 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodriguez, A., Muñoz, E., Jiménez, R. et al. Kinetics of oxidation of iodide by hexacyanoferrate(II) in binary aqueous solvent mixtures. Transition Met Chem 16, 102–107 (1991). https://doi.org/10.1007/BF01127881
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01127881