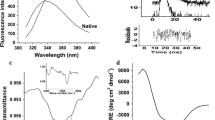
Abstract
Cathepsin D occurs in two forms, a single polypeptide chain (Mr 44000) and a non-covalent complex of two peptides of Mr 14000 and 30000 that is derived by proteolytic processing of the 44000 polypeptide. The two forms from bovine spleen are closely similar in secondary structure content, in aromatic amino acid environment and in the two step denaturation behaviour. Enzyme activity is lost irreversibly on denaturation but conformation can be partially regained. The two separated chains will only refold partially and this is related to their positions in the overall structure of cathepsin D. It is suggested that the processing step is related to protein turnover.
Similar content being viewed by others
References
Andreeva NA & Gustchina AE (1979) Biochem. Biophys. Res. Commun.87, 32–42.
Barrett AJ (1979) Adv. Exp. Med. Biol.95, 291–300.
Barrett AJ & McDonald JK (1980)Mammalian Proteases: A Glossary and Bibliography, vol. 1, pp 338–350, Academic Press, London.
Blundell TL, Sewell BT & McLachlan A (1979) Biochim. Biophys. Acta580, 24–31.
Erickson AH & Blobel G (1979) J. Biol. Chem.254, 11771–11774.
Erickson AH, Conner GE & Blobel G (1981) J. Biol. Chem.256, 11224–11231.
James MNG, Sielecki A, Salituro F, Rich DH & Hofmann T (1982) Proc. Natl. Acad. Sci. U.S.A.79, 6137–6141.
Keilova H & Tomasek V (1976) in:Intracecullar Protein Catabolism (Hanson H & Bohley P, eds), pp 237–251, J. A. Barth, Leipzig.
Kregar I, Urh I, Umezawa H & Turk V (1977) Croat. Chem. Acta49, 587–592.
Laemmli UK (1970) Nature (London)227 680–685.
Lah T & Turk V (1982) Hoppe-Seyler's Z. Physiol. Chem.363, 247–254.
Lah T, Turk V & Pain RH (1980) Vestn. Slov. Kem. Drus.27, 237–250.
Lah T, Drobnic-Kosorok M, Turk V & Pain RH (1984) Biochem. J.218, 601–608.
Misono KS, Chang J-J & Inagami T (1982) Proc. Natl. Acad. Sci. U.S.A.79, 4863–4867.
Ogunro EA, Ferguson AG & Losch M (1980) Cardiovasc. Res.14, 254–260.
Pain RH, Lah T & Turk V (1985) Trans. Biochem. Soc. (in press).
Panthier JJ, Fook S, Chambraud B, Strosberg AD, Corvol P & Rougeon F (1982) Nature (London)298, 90–92.
Press EM, Porter RR & Cebra J (1960) Biochem. J.74, 509–513.
Privalov PL, Mateo PL, Khechinashvilli NN, Stepanov VM & Revina LP (1981) J. Mol. Biol.152, 445–464.
Puizdar V & Turk V (1981) FEBS Lett.132, 299–304.
Shewale JG & Tang J (1984) Proc. Natl. Acad. Sci. U.S.A.81, 3703–3707.
Takahashi T & Tang J (1981) Methods Enzymol.80, 565–581.
Turk V, Lah T, Puizdar V, Kregar I & Pain RH (1981) Acta Med. Ger.40, 1439–1450.
Whitaker JN (1981) Comp. Biochem. Physiol. B68, 215–220.
Yamamoto K, Katsuda N, Himeno M & Kato K (1979) Eur. J. Biochem.95, 459–467.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pain, R.H., Lan, T. & Turk, V. Conformation and processing of cathepsin D. Biosci Rep 5, 957–967 (1985). https://doi.org/10.1007/BF01119908
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01119908