Abstract
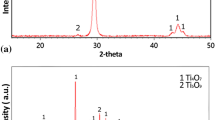
The preparation of aqueous titanium isopropoxide-hydrogen peroxide solutions, their light absorption spectra, optical density changes based on the transformation to colloids, thermal analysis of the dry colloids to titanium dioxide and the spontaneous explosions of the residues produced after vacuum evaporation of the solutions transforming titanium dioxide powders, were studied. The solutions were prepared by dissolving titanium tetra isopropoxide in an aqueous hydrogen peroxide solution. Component isopropanol was expelled from the mixture by vacuum evaporation. The presence of various H2O2/Ti ratios of residues produced by the evaporation, and the variation of absorption spectra with the concentration of hydrogen-peroxide suggested that the hydrogenperoxide content in the solute species was determined by the concentration of hydrogen peroxide in them. The thermal analysis of the dry colloids revealed that they suddenly decompose exothermally and consist of titanium, water and hydrogenperoxide. X-ray powder diffraction revealed that the dry colloid was amorphous and the heated product of the colloid at 440°C was in the anatase form and also that the explosion products were the same. SEM revealed that the fine dry colloid deformed above 609°C.
Similar content being viewed by others
References
W. B. Blumenthal,Ceram. Age 51 (1948) 320.
Schoenn,Z. Anal. Chem. 9 (1870) 41.
Idem, ibid. 9 (1870) 330.
Edward Jackson,Chem. News 47 (1883) 157.
OscarMilner, Kenneth L. Proctor andSidney Weinberg,Ind. Eng. Chem. Anal. Ed. 17 (1945) 142.
Alfred Weissler,ibid. 17 (1945) 695.
Idem, ibid. 17 (1945) 775.
G. Duyckaerts,Anal. Chim. Acta 2 (1948) 662.
F. C. Palilla, Norman Adler andC. F. Hiskey,Anal. Chem. 25 (1953) 926.
Philip B. Sweetser andClark E. Bricker,ibid. 26 (1954) 195.
W. T. L. Neal,Analyst 79 (1954) 403.
G. W. C. Milner andP. J. Phennah,ibid. 79 (1954) 414.
Sir A. C. Egerton, A. J. Everett, G. J. Minkoff, S. Rudrakanchana andK. C. Salooja,Anal. Chim. Acta. 10 (1954) 422.
Dadid Lewis,J. Phys. Chem. 62 (1958) 1145.
William G. Scribner,Anal. Chem. 32 (1960) 966.
Marie-Ellsa Rumpf,Compt. Rend. 200 (1935) 317.
Idem, Ann. Chim. 8 (1937) 456.
Maximiliane Bendig andH. Hirschmueller,Z. Anal. Chem. 120 (1940) 385.
M. E. Rump,Bull. Soc. Chim. France 12 (1945) 283.
Yvonne Schaeppi andW. D. Treadwell,Helv. Chim. Acta. 31 (1948) 577.
V. P. Vasil'ev andP. N. Vorob'ev,J. Anal. Chem. USSR 22 (1967) 613.
A. Ringbom, “Complexation in Analytical Chemistry” (Wiley, New York, 1963) trans. to Japanese by Sangyo Tosho Pub. Co. Ltd.
R. Schwarz andW. Sexauer,Ber. 60 (1927) 500.
M. Shiota, A. Sato, M. Tsutsumi, K. Mura Matsu andS. Honma, The Chemical Society of Japan 53rd annual autumn meeting (1986) 1R19, p. 817.
Walter Mahler andMax. F. Bechtold,Nature 285 (1980) 27.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shiota, M. Some properties of aqueous titanium isopropoxide-hydrogenperoxide solutions and their decomposition to produce titanium dioxide. J Mater Sci 23, 1718–1724 (1988). https://doi.org/10.1007/BF01115711
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01115711