Abstract
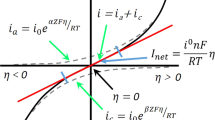
The determination of the state-of-charge of the lead-acid battery has been examined from the viewpoint of internal impedance. It is shown that the impedance is controlled by charge transfer and to a smaller extent by diffusion processes in the frequency range 15–100 Hz. The equivalent series/parallel capacitance as well as the a.c. phase-shift show a parabolic dependence upon the state-of-charge, with a maximum or minimum at 50% charge. These results are explained on the basis of a uniform transmission-line analog equivalent circuit for the battery electrodes.
Similar content being viewed by others
Abbreviations
- Battery:
-
This word is used synonymous with the word ‘cell’
- R p :
-
equivalent parallel resistance (Ω)
- R s :
-
equivalent series resistance (Ω)
- ¦Z¦:
-
modulus of impedance (Ω)
- C p :
-
equivalent parallel capacitance (F)
- C s :
-
equivalent series capacitance (F)
- φ :
-
a.c. phase-shift (radians or degrees)
- ω :
-
2πf
- f :
-
a.c. frequency (Hz)
- R Ω :
-
resistance of electrolyte solution and separator (Ω)
- ¯C :
-
double layer capacity (F)
- W :
-
diffusional (Warburg) impedance (Ω)
- R t :
-
resistance due to polarization (Ω)
- α :
-
energy transfer coefficient
- T :
-
absolute temperature (K)
- R :
-
gas constant
- F :
-
Faraday constant
- C 0O :
-
bulk concentration of the oxidant
- C 0R :
-
bulk concentration of the reductant
- D O :
-
diffusion coefficient of the oxidant
- D R :
-
diffusion coefficient of the reductant
- σ :
-
Warburg coefficient
- N :
-
number of pores/area
- A :
-
active area of the electrode (cm2)
- S :
-
state-of-charge
- a:
-
anode
- c:
-
cathode
- L :
-
inductance
- I o :
-
exchange current
References
S. Sathyanarayana, S. Venugopalan and M. L. Gopikanth,J. Appl. Electrochem. 9 (1979) 125.
S. Sathyanarayana, S. Venugopalan and M. L. Gopikanth,J. Appl. Electrochem. 8 (1978) 479.
M. Sluyters-Rehbach and J. H. Sluyters, ‘Electroanalytical Chemistry’, (Edited by A. J. Bard) Vol. 8, Marcel Dekker Inc. (1971) p. 157.
S. Sathyanarayana,Trans. Soc. Adv. Electrochem. Sci. Tech. 11 (1976) 19.
Idem, unpublished work.
B. E., Conway and P. L. Bourgault,Canad. J. Chem. 37 (1959) 292.
Idem, ibid 40 (1962) 1690.
R. de Levie, ‘Advances in Electrochemistry and Electrochemical Engineering’, (Edited by P. Delahay and C. W. Tobias) Vol. 6, Interscience (1967) p. 329.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gopikanth, M.L., Sathyanarayana, S. Impedance parameters and the state-of-charge. II. Lead-acid battery. J Appl Electrochem 9, 369–379 (1979). https://doi.org/10.1007/BF01112492
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01112492