Abstract
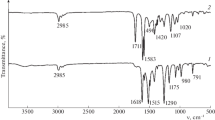
On the basis of an analysis of published data, it is suggested that the affinity of cations for a silica surface should be determined not only by electrostatic forces but also by the nature of the electron density distribution between the silicon atoms and the metal ion in the surface M+ SiO− groups. This suggestion was tested and qualitatively confirmed by experiments on the sorption of cations of the elements of the main subgroups I–III of the periodic system on the calcium (from solutions of the nitrates) and hydrogen (from solutions of the hydroxides) forms of silica gel, and also by the results of a study of the infrared spectra of a number of cation-substituted forms of this sorbent in the range 700–1500 cm−1.
Similar content being viewed by others
References
M. Milone and G. Getini, Atti accad., Torino,90, 3, 1955–1956; G. Cetini and F. Ricca, Atti accad., Torino,90, 229, 1955–1956.
S. Ahrland, I. Grenthe, and B. Noren, Acta Chem. Scand.,14, 1059, 1077, 1960.
E. V. Egorov and P. D. Novikov, The Action of Ionizing Radiation on Ion-Exchange Materials [in Russian], Atomizdat, Moscow, 1965.
R. K. Iler, Colloid Chemistry of Silica and Silicates [Russian translation], Gosstroiizdat, Moscow, 1959.
V. V. Gromov and V. I. Spitsyn, Atomnaya energiya,14, 491, 1963.
D. N. Strazhesko and G. F. Yankovskaya, Ukr. khim. zhurnal,25, 471, 1959.
A. P. Dushina and V. B. Aleskovskii, Silica Gel—An Inorganic Cation Exchanger [in Russian], Goskhimizdat, Leningrad, 1963.
V. Markova and F. Vydra, Chem. listy,60, 860, 1966.
L. F. Kirichenko and Z. Z. Vysotskii, DAN SSSR,175, 635, 1967.
M. T. Rogers and R. Van der Vernen, J. Amer. Chem. Soc.,75, 1751, 1953.
C. M. French and J. P. Howard, Trans. Farad. Soc.,52, 712, 1956.
D. L. Dugger, J. H. Stanton, B. N. Irby, B. L. McConnell, W. W. Cummings, and R. W. Maatman, J. Phys. Chem.,68, 754, 1964.
F. Helfferich, Ion Exchange [Russian translation], IL, Moscow, 1962.
M. G. Voronkov, collection: The Chemistry and Practical Application of Organosilicon Compounds [in Russian], p. 136, 1961.
A. N. Lazarev, Izv. AN SSSR, ser. khim., 235, 1964.
A. N. Lazarev, collection: Structural Changes in Glasses at Elevated Temperatures [in Russian], Nauka, Moscow-Leningrad, p. 233, 1966.
D. W. J. Cruickshank, J. Chem. Soc., 5486, 1961.
O. Samuelson, Ion-Exchange Separations in Analytical Chemistry [Russian translation], Khimiya, Moscow-Leningrad, p. 67, 1966.
F. E. Bartell and Y. Fu, J. Phys. Chem.,33, 676, 1929.
B. B. Damaskin, N. V. Nikolaeva-Fedorovich, and A. N. Frumkin, DAN SSSR,121, 129, 1958.
L. S. Ivanova and D. N. Strazhesko, DAN UkrSSR,8, 869, 1959.
I. B. Slinyakova, G. B. Budkevich, and I. E. Neimark, Kollodn. zhurnal,27, 758, 1965.
R. Kopff, R. Rueff, and H. Benoit, Bull. Soc. chim. France,10, 1694, 1960.
V. A. Florinskaya and R. S. Pechenina, Izv. AN SSSR, ser. fiz.,17, 649, 1953.
I. A. Sevchenko and V. A. Florinskaya, Opt. i spektr.,4, 189, 1958.
J. Zarzycki and F. Naudin, J. chim. phys. et phys. chim. biol.,58, 830, 1961.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubanik, S.K., Baran, A.A., Strazhesko, D.N. et al. Selective adsorption of cations of groups I, II, and III of the periodic system on various ion-exchange forms of silica gel. Theor Exp Chem 5, 232–235 (1969). https://doi.org/10.1007/BF01109669
Issue Date:
DOI: https://doi.org/10.1007/BF01109669