Conclusions
-
1.
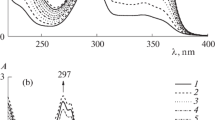
A study has been made of the acid-base properties of the five- and six-membered phosphates, phosphonates, and phosphinates. Thermodynamic spectral characteristics of the H-complexes with p-fluorophenol have been measured in CCl4, solution; using the method of potentiometric titration with HClO4, the pK a (CH3NO2) values have been obtained for the more basic of these compounds.
-
2.
General linear correlation equations have been obtained for the H-complexes of p-fluorophenol with cyclic and acyclic phosphoryl compounds.
-
3.
The σP values have been calculated for the phosphor ring fragments. The difference between the σP constants for the five- and six-membered rings remains unchanged in moving through the series of compounds studied here. Passage from a six-membered to a five-membered cycle reduced the proton-acceptor activity of the phosphoryl group in every case.
Similar content being viewed by others
Literature cited
E. I. Matrosov, A. A. Kryuchkov, É. E. Nifant'ev, and M. I. Kabachnik, Izv. Akad. Nauk SSSR, Ser. Khlm., 530 (1976).
G. Aksnes and T. Gramstad, Acta Chem. Scand.,14, 1485 (1960).
T. Gramst'ad, Acta Chem., Scand.,15, 1337 (1961).
T. Gramstad and W. I. Fuglevick, Acta Chem. Scand.,16, 2368 (1962).
T. Gramstad and A. Shaprud, Acta Chem. Scand.,16, 999 (1962).
U. Blindheim and T. Gramstad, Spectrochim. Acta,21, 1073 (1965).
U. Blindheim and T. Gramstad. Spectrochim. Acta,25A, 1105 (1969).
P. Haake, R. D. Cook and G. H. Hurst, J. Am. Chem. Soc.,89, 2650 (1967).
A. G. Cook and G. W. Mason, J. Inorg. Nucl. Chem.,35, 2090 (1973).
N. K. Skvortsov, G. F. Tereshchenko, B. I. Ionin, and A. P. Petrov, Zh. Obshch. Khim.,43, 981 (1973).
B. I. Stepanov, B. A. Korolev, and A. I. Bokanov, Zh. Obshch. Khim.,39, 316 (1969).
E. I. Matrosov, E. N. Tsvetkov, Z. N. Mironova, R. A. Malevannaya, and M. I. Kabachnik, Izv. Akad. Nauk SSSR, Ser. Khim., 1333 (1975).
R. Pujol, P.-P. Majoral, J. Navech, and F. Mathis, C. R. Acad. Sci.,274B, 66 (1972).
G. Aksnes and P. Albriktsen, Acta Chem. Scand.,22, 1866 (1968).
L. J. V. Griend, D. W. White and J. G. Verkade, Phosphorus,3, 5 (1973).
E. I. Matrosov, A. G. Kozachenko, and M. I. Kabachnik, Izv. Akad. Nauk SSSR, Ser. Khim., 313 (1976).
B. N. Laskorin, V. V. Yakshin, E. P. Buchikhin, L. I. Sokal'skaya, and V. I. Medvedev, Teor. Eksp. Khim.,9, 245 (1973).
C. A. Streuli, Anal. Chem.,31, 1652 (1959).
T. Gramstad, Acta Chem. Scand.,16, 807 (1962).
T. A. Mastryukova and M. I. Kabachnik, Usp. Khim.,38, 1751 (1969).
E. M. Arnett, R. P. Quirk, and J. W. Larsen, J. Am. Chem. Soc.,92, 3977 (1970).
L. S. Khaikin and L. V. Vilkov, Usp. Khim.,41, 2224 (1972).
G. M. Blackburn, J. S. Cohen, and L. Weatherall, Tetrahedron,27, 2903 (1971).
R. L. Collin, J. Am. Chem. Soc.,88, 3281 (1966).
D. B. Boyd, J. Am. Chem. Soc.,91, 1200 (1969).
B. V. Rassadin and A. V. Iogansen, Zh. Prikl. Spektrosk.6, 801 (1967).
V. V. Korshak, I. A. Gribova, and M. A. Andreeva, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 631 (1957).
L. Keay and E. M. Crook, J. Chem. Soc., 710 (1961).
A. Y. Garner, USA Patent 2916510, 1959; Chem. Abstrs.,54, 5571 (1960); USA Patent 2953591 (1960); Chem. Abstrs.,55, 5346 (1961).
B. A. Arbuzov and D. Kh. Yarmukhametova, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 1767 (1960).
B. Helferich and E. Aufderhaar, Liebigs Ann. Chem.,658, 100 (1962).
C. Aksnes and K. Bergesen, Acta Chem. Scand.,20, 2508 (1966).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 4, pp. 791–797., April, 1977.
Rights and permissions
About this article
Cite this article
Matrosov, E.I., Kryuchkov, A.A., Nifant'ev, É.E. et al. Electronic effects in cyclic phosphates, phosphonates, and phosphinates. Russ Chem Bull 26, 719–725 (1977). https://doi.org/10.1007/BF01108188
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01108188