Abstract
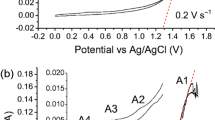
The anodic oxidation of tin in 0.1 to 1M bicarbonate solutions at pH 8 has been studied. The process may be divided into three potential regions: (1) a short active dissolution (Tafel) region; (ii) a dissolution-precipitation region; and (iii) a large region of electrode passivity. The rate-determining step of the reaction in the active-dissolution region is attributed to the diffusion of an ionic species into the solution, the diffusing species being generated at the metal surface. In the region of the first oxidation peak, the reaction rate is controlled by diffusion of CO 2−3 species in solution. When the potential becomes more positive than −0,1 Vsce, a highly passivating (most likely SnO2) film is formed on the electrode surface.
Similar content being viewed by others
References
S. N. Shah, D. E. Davies,Electrochim. Acta 8 (1963) 663.
A. M. Shams El Din, F. M. Abd El Wahad,9, (1964) 883.
S. A. Awad, A. Kassab,J. Electroanal. Chem. 20 (1969) 203;26 (1970) 127.
B. N. Stirrup, N. A. Hampson,67 (1976) 45, 57.
T. Dickinson, S. Lotfi,Electrochim. Acta 23 (1978) 513, 995.
H. Do Duc, P. Tissot,J. Electroanal. Chem. 102 (1979) 59.
Corrosion Sci.,19 (1979) 179.
V. S. Muralidharan, K. Thangavel, K. S. RajagopalanElectrochim. Acta 28 (1983) 703.
R. O. Ansell T. Dickinson, A. F. Povey, P. M. A. Sherwood,J. Electrochem. Soc. 124 (1977) 1360.
T. D. Burleigh, H. Gerischer,135 (1988) 2938.
D. E. Davies, S. N. Shah,Electrochim. Acta 8 (1963) 703.
S. D. Kapusta, N. Hackerman,25 (1980) 1001, 1625.
E. Deltombe, N. de Zoubov, M. Pourbaix, Rapport Tech. R. T. 25 du CEBELCOR (1955).
M. Pourbaix, ‘Atlas of Electrochemical Equilibria in Aqueous Solutions’, 2nd ed., NACE Houston, USA (1974) p. 475.
C. I. House, G. H. Kelsall,Electrochim. Acta 29 (1984) 1459.
W. M. Latimer, ‘Oxidation Potentials’, 2nd ed., Prentice-Hall, New Jersey (1952).
Z. Galus, in ‘Encyclopedia of the Electrochemistry of the Elements’ (edited by A. J. Bard), vol. 4, Dekker, New York (1975).
‘Standard Potentials in Aqueous Solution’ (edited by A. J. Bard, R. Parsons and J. Jordan), Dekker, New York (1985).
R. Gilbert, Tech. Rapport IREQ-4150b, Institut de Recherche d'Hydro-Québec, Varennes (Qué), April 1988.
V. G. Levich, ‘Physiochemical Hydrodynamics’ Prentice-Hall, Englewood Cliffs, New York (1966).
G. Charlot, ‘L' Analyse quantitative et les réactions en solution’, 4th ed., Masson, Paris (1957).
A. J. Bard, L. R. Faulkner, ‘Electrochemical Methods’, John Wiley & son, New York (1980).
R. Parson, ‘Handbook of Electrochemical Constants’, Butterworths, London (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drogowska, M., Ménard, H. & Brossard, L. Electrochemical behaviour of tin in bicarbonate solution at pH 8. J Appl Electrochem 21, 84–90 (1991). https://doi.org/10.1007/BF01103835
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01103835