Abstract
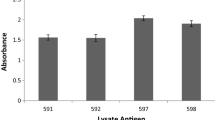
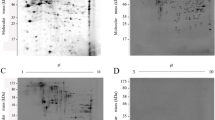
Water-soluble antigens liberated from the disrupted mycelium of nine dermatophytes (seven isolates ofMicrosporum canis, one each ofMicrosporum gypseum andTrichophyton mentagrophytes) were compared by analytical slab SDS-PAGE. No substantial differences were observed between the protein bands of theM. Canis isolates, but certain distinctive bands were apparent in the other two species examined. Western immunoblotting usingM. canis-derived antigens separated by SDS-PAGE was used to investigate the humoral immune response in 79 cats with naturally-occurring dermatophytosis (72 withM. canis, six withM. gypseum and one withT. mentagrophytes) and this information was compared to results of immunoblots from 46 control (non-dermatophyte exposed) cats. Seven dominant bands (bands which occurred frequently and stained heavily) were identified in immunoblots from the dermatophyte-infected cats with apparent molecular weights varying between 39 and 120 kD. None of these bands were totally specific markers for dermatophytosis as a variable proportion of the control cats showed reactivity to all these proteins. However, most (73%) of the dermatophyte-infected cats showed reactivity to six or seven of the identified bands whereas most (80%) of the control cats showed reactivity to between zero and three of these bands (p<0.005). Western immunoblotting could be used to select individual immunodominant antigens for further evaluation of protective (cell-mediated) immunity.
Similar content being viewed by others
References
Muller GH, Kirk RW, Scott DW. Fungal disease. In: Small animal dermatology, 4th ed. Philadelphia: WB Saunders, 1989: 295–346.
DeBoer DJ, Moriello KA. Humoral and cellular immune responses toMicrosporum canis in naturally occurring feline dermatophytosis. J Med Vet Mycol 1993; 31: 121–32.
Sparkes AH, Stokes CR, Gruffydd-Jones TJ. Humoral immune responses in cats with dermatophytosis. Am J Vet Res 1993; 54: 1869–73.
Calderon RA, Hay RJ. Cell-mediated immunity in experimental murine dermatophytosis, II: Adoptive transfer of immunity to dermatophyte infection by lymphoid cells from doners with acute or chronic infections. Immunology 1984; 53: 465–72.
Green F, Weber JK, Balish E. The thymus dependency of acquired resistance toTrichophyton mentagrophytes dermatophytosis in rats. Invest Dermato 1983; 81: 31–38.
Blake JS, Dahl MV, Herron MJ, Nelson RD. An immunoinhibitory cell wall glycoprotein (mannan) fromTrichophyton rubrum. J Invest Dermatol 1991; 96: 657–61.
Johnstone A, Thorpe R. Polyacrylamide gel techniques. In: Johnstone A, Thorpe R, eds. Immunochemistry in practice, 2nd ed. Oxford: Blackwell, 1987: 148–82.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–85.
Polonelli L, Morace G. Serological procedures to detect dermatophyte antigens. In: Kurstak E, ed. Immunology of fungal diseases. New York: Marcel Dekker, 1989; 419–57.
Shechter Y, Landau JW, Dabrowa N, Newcomer VD. Comparative disc electrophoretic studies of proteins from dermatophytes. Sabouraudia 1966; 5: 144–49.
Christiansen AH, Svejgaard E. Studies of the antigenic structure ofTrichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis, andEpidermophyton floccosum by crossed immuno-electrophoresis. Acta Path Microbiol Scand, Sect C 1976; 84: 337–41.
Philpot CM. Serological differences among the dermatophytes. Sabouraudia 1978; 16: 247–56.
de Montolos H, Guinet RMF. Antigenic structure of dermatophytes as demonstrated by quantitative immunoelectrophoresis. Mykosen 1982; 25: 705–10.
Hearn VM, MacKenzie DWR. The preparation and partial purification of fractions from mycelial fungi with antigenic activity. Mol Immunol 1980; 17: 1097–1103.
Holden CA, Hay RJ, MacDonald DM. A method for identification of dermatophyte antigens in situ by an immunoperoxidase technique in light and electron microscopy. Clin Exp Immunol 1981; 6: 311–16.
de Haan P, van der Raay-Helmer EMH, Boorsma DM. Diversity of antigenic extracts from the dermatophyteTrichophyton rubrum. Mykosen 1987; 30: 427–33.
Garg AP, Muller J. Preparation of antigens fromTrichophyton mentagrophytes using a new semi-solid culture medium and their characterization by SDS-PAGE and immunological techniques. Mycoses 1992; 35: 349–55.
Tucker WDL, Noble WC. The value of electrophoretic protein patterns for the study ofMicrosporum canis. J Med Vet Mycol 1990; 28: 117–23.
Tucker WDL, Noble WC. Polyacrylamide gel electrophoresis patterns of someMicrosporum species. Mycoses 1991; 34: 303–7.
Symoens F, Willenz Ph, Rouma V, Planard C, Nolard N. Isoelectric focusing applied to taxonomic differentiation of theTrichophyton mentagrophytes complex and the relatedTrichophyton interdigitale. Mycoses 1989; 32: 652–63.
de Haan P, Wikler JR, van der Raay-Helmer EMH, Boorsma DM. Antigens of dermatophytes and their characterization using monoclonal antibodies. In: Kurstak E, ed. Immunology of fungal diseases. New York: Marcel Dekker, 1989: 113–32.
Walzer RA, Einbinder J. Immunofluorescent studies in dermatophyte infection. J Invest Dermatol 1962; 39: 165–68.
Kaaman T, von Stedingk LV, von Stedingk M, Wasserman J. ELISA-determined serological reactivity against purified trichophytin in dermatophytosis. Acta Derm Venereol 1981; 61: 313–17.
Hay RJ, Shennan G. Chronic dermatophyte infections, II: Antibody and cell-mediated immune responses. Br Dermatol 1982; 106: 191–98.
Honbo S, Jones HE, Artis WM. Chronic dermatophyte infection: evaluation of the Ig class-specific antibody response reactive with polysaccharide and peptide antigens derived fromTrichophyton mentagrophytes. J Invest Dermatol 1984; 82: 287–90.
Roig AM, Rodriguez JMT. The immune response in childhood dermatophytoses. Mykosen 1987; 30: 574–80.
Lee KH, Kim YA, Lee MG, Lee JB. Detection of IgG and IgM antibodies to purified keratinolytic proteinase in sera from patients with dermatophytosis by enzyme-linked immunosorbent assay. Ann Dermatol 1989; 1: 1–5.
Pepys J, Riddel RW, Clayton YM. Human precipitins against common pathogenic and non-pathogenic fungi. Nature 1959; 184: 1328–29.
Svejgaard E, Christiansen AH. Precipitating antibodies in dermatophytosis demonstrated by crossed immunoelectrophoresis. Acta Path Microbiol Scand, Sect C 1979; 87: 23–27.
Cox WA, Moore JA. ExperimentalTrichophyton verrucosum infections in laboratory animals. J Comp Path 1968; 78: 35–41.
Grappel SF, Bishop CT, Blank F. Immunology of dermatophytes and dermatophytosis. Bacteriol Rev 1974; 38: 222–50.
Basarab O, How MJ, Cruickshank CND. Immunological relationships between glycopeptides ofMicrosporum canis, Trichophyton rubrum, Trichophyton mentagrophytes and other fungi. Sabouraudia 1968; 6: 119–26.
Jones HE. Immune response and host resistance of humans to dermatophyte infection. J Am Acad Dermatol 1993; 28: S12-S18.
Sparkes AH, Stokes CR, Gruffydd-Jones TJ. Correlation between immunological and clinical observations during experimentalM. canis infection in cats (Abstract). J Vet Int Med 1994; 8: 171.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sparkes, A.H., Stokes, C.R. & Gruffydd-Jones, T.J. SDS-PAGE separation of dermatophyte antigens, and western immunoblotting in feline dermatophytosis. Mycopathologia 128, 91–98 (1994). https://doi.org/10.1007/BF01103015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01103015