Abstract
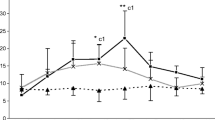
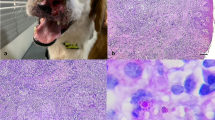
We compared the granuloma morphology and immune response of hamsters inoculated withParacoccidioides brasiliensis (Pb) into the cheek pouch, which lacks lymphatic drainage, and into the footpad, which is rich in lymphatics. Our objective was to better understand the modulation ofPb granuloma in an immunocompetent animal inoculated in an immunologically privileged site. The humoral immune response (ELISA) and cell mediated immunity (footpad test) became positive on days 7 and 14, respectively in animals inoculated into footpad and on days 35 and 60 in animals inoculated into the pouch. Typical epithelioid granulomas were observed at both sites on day 14. The number of fungi gradually decreased from the beginning of the experiment in footpad lesions, but only after day 35 in pouch granulomas, when cell mediated immunity was detectable. The results indicate that typical epithelioid paracoccidioidomycotic granulomas may develop in the absence of a detectable immune response; however, they are incapable of controlling fungal reproduction. Lack of lymphatic drainage delays the appearance of a detectable immune response, but with time fungi escape from the pouch, elicit an immune response and reach other organs. Our results further indicate the importance of the lymphatics in the pathogenesis of paracoccidioidomycosis.
Similar content being viewed by others
Abbreviations
- HCP:
-
hamster cheek pouch
- Pb :
-
Paracoccidioides brasiliensis
- Pbmycosis:
-
Paracoccidioidomycosis
References
Franco MF, Mendes RP, Moscardi-Bacchi M, Rezkallah-Iwasso MT, Montenegro MR. Paracoccidioidomycosis. In: Hay RJ, ed. Tropical fungal infection. London: Bailliere Tindall, 1989: 185–220.
Robledo MA, Graybill JR, Ahrens J, Robledo A, Drutz DJ, Robledo M. Host defense against experimental paracoccidioidomycosis. Am Rev Resp Dis 1982; 125: 563–567.
Kerr IB, Mendes da Silva AM, Drouhet E, Oliveira P, Costa SC. Paracoccidioidomycosis in nude mice: presence of filamentous forms of the fungus. Mycopathologia 1988; 101: 3–11.
Miyaji M, Nishimura K. Granuloma formation and killing functions of granuloma in congenitally athymic nude mice infected withBlastomyces dermatitides andParacoccidioides brasiliensis. Mycopathologia 1983; 82: 129–141.
Suya H, Fujioka A, Pincelli C, Fukuyama K, Epstein W. Skin granuloma formation in mice immunosupressed by cyclosporine. J Invest Dermatol 1988; 90: 430–433.
Barker F & Billigham RE. Immunologically privileged sites. Adv Immunol 1977; 25: 1–54.
Fava-Netto C, Vegas VS, Sciannamea IM, Guarniere DB. Antígeno polissacarídico doParacoccidioides brasiliensis. Estudo do tempo de cultivo doP. brasiliensis necessário ao preparo do antígeno. Rev Inst Med Trop São Paulo 1969; 11: 177–181.
Calich VLG, Purchio A, Paulo CR. A new fluorescent viability test for fungi cells. Mycopathology 1978; 66: 175–177.
Silva MIC, Carvalho M, Franco TN, Cunha MA, Chamma LG, Fogaça J, Fecchio D, Franco MF. The use of a mixture of somatic and culture filtrate antigens in the evaluation of response toParacoccidioides brasiliensis. J Med Vet Mycol 1991; 29: 1–4.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275.
Kong YM, Savage D, Kong LNL. Delayed dermal hypersensitivity in mice to spherule and mycelial extracts ofCoccidioides immitis. J Bacteriol 1966; 91: 876–883.
Mendes-Giannini MJS, Camargo ME, Lacaz CS, Ferreira AW. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidomycosis. J Clin Microbiol 1984; 20: 103–108.
Hsu SM, Raine L, Fanger N. The use of anti-avidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase techiniques. Am J clin Path 1981; 75: 816–821.
Peraçoli MTS, Mota NGS, Montenegro MRG. Experimental paracoccidioidomycosis in the syrian hamster. Morphology and correlation of lesions with humoral and cell-mediated immunity. Mycopathologia 1982; 79: 7–17.
Rezkallah-Iwasso MT, Mota NGS, Gomes MCG, Montenegro MRG. Effect of levamisole on experimental paracoccidioidomycosis in the syrian hamster: immunologic and histopathologic correlation. Mycopathologia 1983; 84: 171–180.
Shepro D, Cohen PS, Kula N. The incompetence of the hamster cheek pouch membrane as a mechanical barrier to the movement of tritiatedE. coli B3 and tritiated T4 bacteriophage. J Immunol 1964; 9: 725–731.
Adams DO. The granulomatous inflamatory response. A review. Am J Pathol 1976; 84: 164–191.
Epstein WL, Furuyama K, Dannok K, Kwan-Wong E. Granulomatous inflammation in normal and athymic mice infected withSchistosoma mansoni: an ultrastructural study. J Pathol 1979; 127: 207–215.
Hoffsten PE, Dixon FJ. Effect of irradiation and cyclophosphamide and anti-K1h antibody formation in mice. J Immunol 1974; 112: 564–572.
North RJ, Izzo AA. Animal model granuloma formation in severe combined immunodeficient (SCID) mice in response to progressive BCG infection. Tendency not to form granuloma in the lung is associated with faster bacterial growth in the organ. Am J Pathol 1993; 142; 1959–1966.
Iabuki K, Montenegro MRG. Experimental paracoccidioidomycosis in the syrian hamster. Morphology, ultrastructure and correlation of lesions with presence of specific antigens and serum levels of antibodies. Mycopathologia 1979; 67: 131–141.
Franco MF, Montenegro MRG. Anatomia patológica. In: Del Negro G, Lacaz CS, Fiorillo AM, eds. Paracoccidioidomicose. Blastomicose sul-americana. São Paulo: Sarvier, 1982: 97–117.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arruda, M.S.P., Coelho, K.I.R. & Montenegro, M.R. Experimental paracoccidioidomycosis of hamster inoculated in the cheek pouch. Mycopathologia 128, 67–73 (1994). https://doi.org/10.1007/BF01103011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01103011